Online Quiz
Learning
Temperature & Heat Transfer
Important terms
Energy ability or capacity to do work, result from motion such as kinetic energy
Matter anything has mass & occupies space
Kietic energy (k) energy from motion
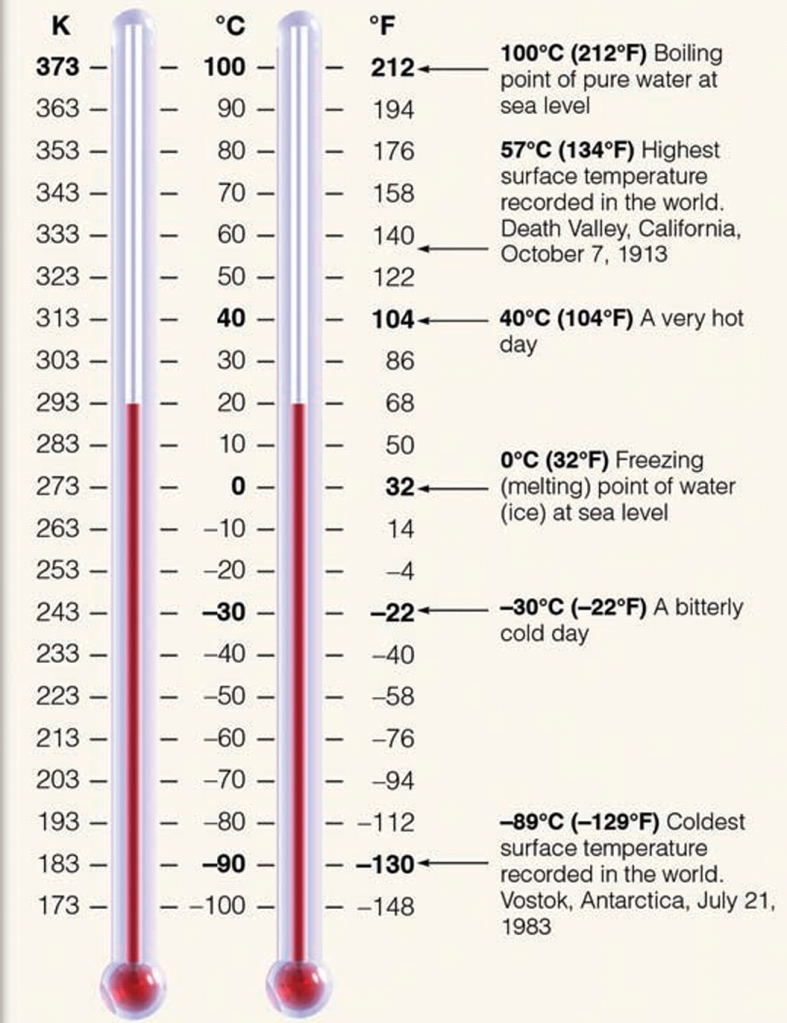
Calorie: amount of heat required to raise T of 1gH₂O from 14.6°C to 15.5°C (1cal = 4.186J)
kilocalorie: is 1000 calories, heat required to raise 1kgH₂O 18°C
Heat capacity: ratio of heat absorbed by substance to its corresponding T rise
C = Ε/ΔΤ [J/°C]
specific heat: heat capacity of a substance per unit mass, or amount of heat needed to raise the T of 1g of a substance 1°C(Ex. If heat 1g of water on a stove, it take 1cal to raise its T 1°C)
S = C/m = E/mΔΤ [J/g°C]
Sensible heat: the heat we can feel, “sense,” & measure with a thermometer
Important concepts
Work is done on matter if pushed, pulled, or lifted over distance
The energy within a body result of its motion, such as Potential & Kinetic energy
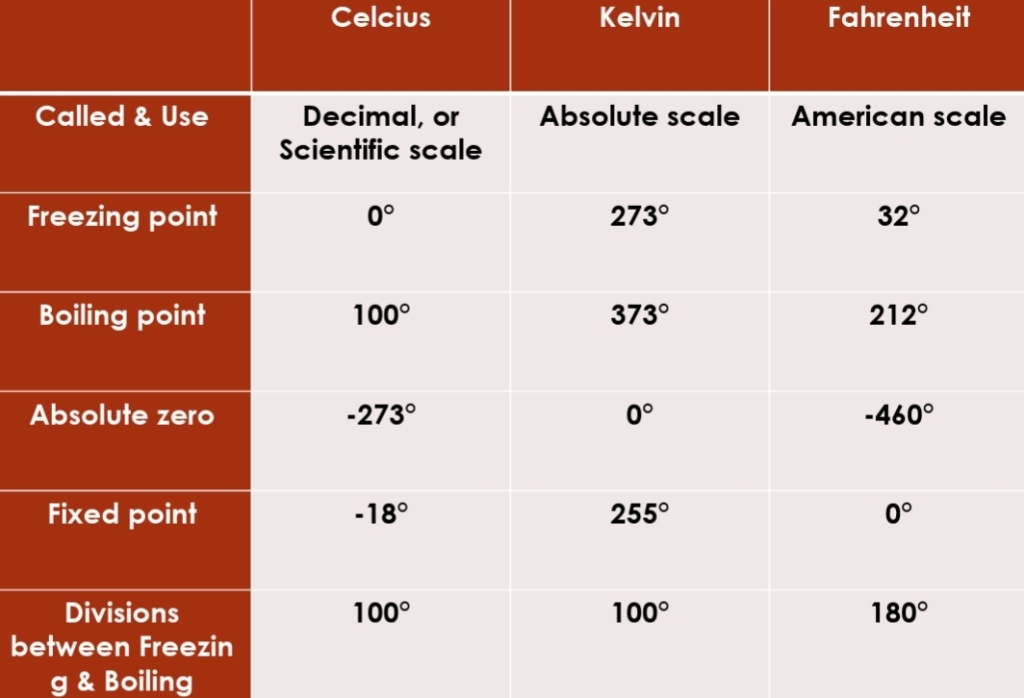
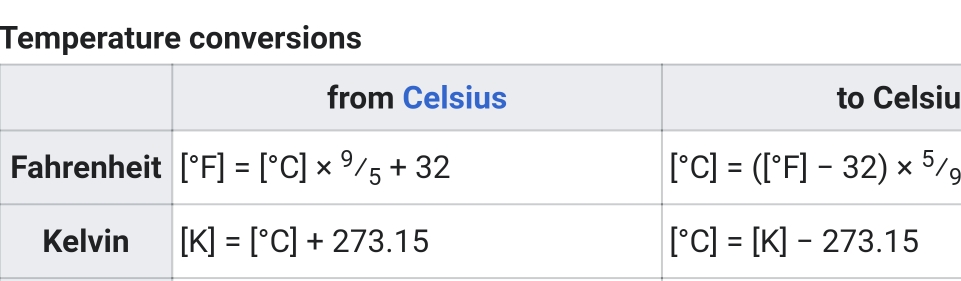
Temperature
average of Kinetic energy or average speed of atoms & molecules (energy within body that result of its motion)
describes how warm or cold an object is

Heat
ENERGY that TRANSFER from one body to another due to Differences in temperature
FLOW OF ENERGY due to Differences in T
After energy transferred it stored as internal energy
Latent Heat
energy required to change state of substance
Ex. H₂O(s) –> H₂O(l), T in these reaction stay constant & Heat is used to MELT the ice doesn’t produce a T change
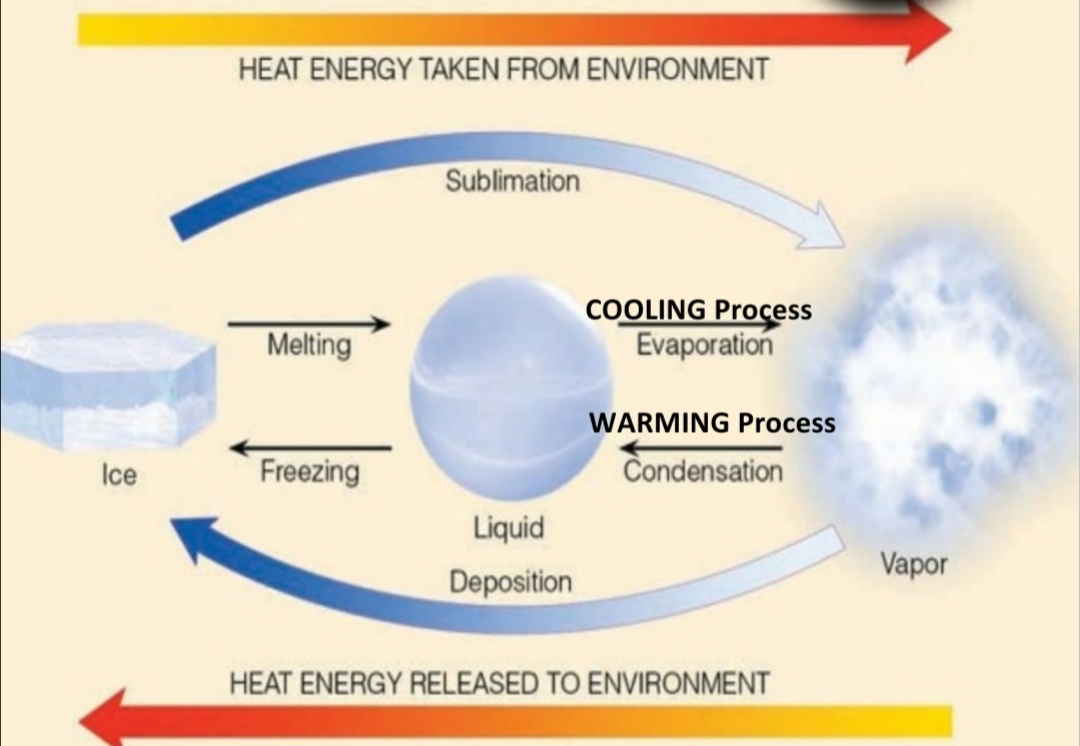
Sublimation: s → g
Evaporation: l → g
Melting: s → l
Energy relesed to environment
Condensation: g → l
Deposition: g → s
Freezing: l → s
COOLING PROCESS: Evaporation
WARMING PROCESS: Condensation
Mechanics of Heat transmission
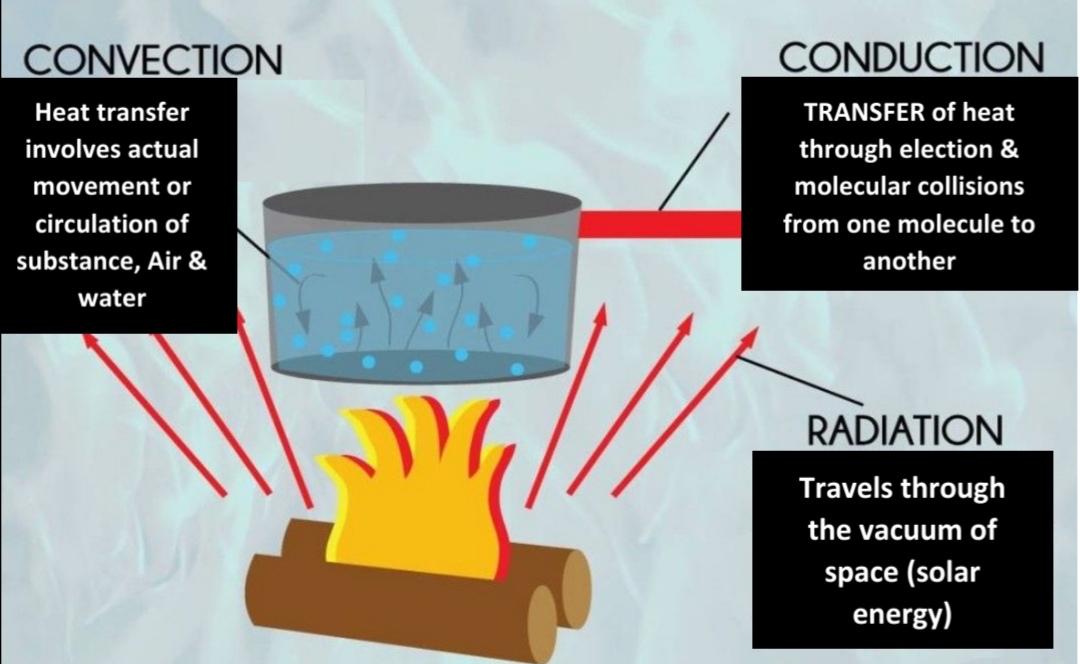
Conduction: heat trensfer involves election & moleculer collisions from one to another
Radiation: heat trensfer via vacum of space
Ability to conduct are varies
Metals are better
Air INSULATOR (not conduct well)
Convection
Most common in atm (Only important for heating air in DIRECT contact with surface)
Thermals: circulation movement
Advection: horizontal movement

Radiation
Wavelengths (λ)
Is a distance between 2 crest
All λ travel at 300,000 km/s
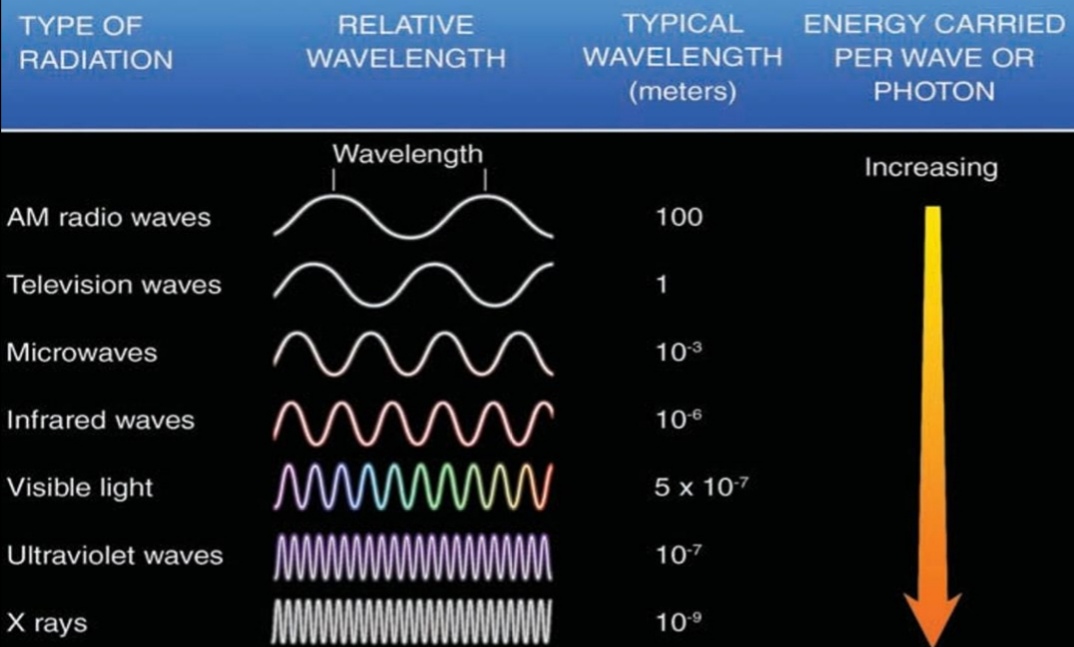
Increase λ –> decrease energy, & frequency
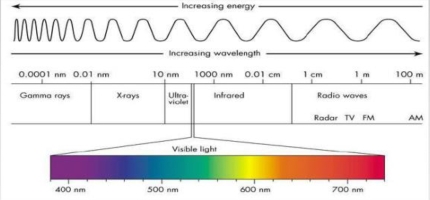
RW > μw > IR > VL > UV > χ-ray > γ-ray
RW: long RW > AM > TV
VL: Red>Orange>Yellow>Green>Blue>Violet
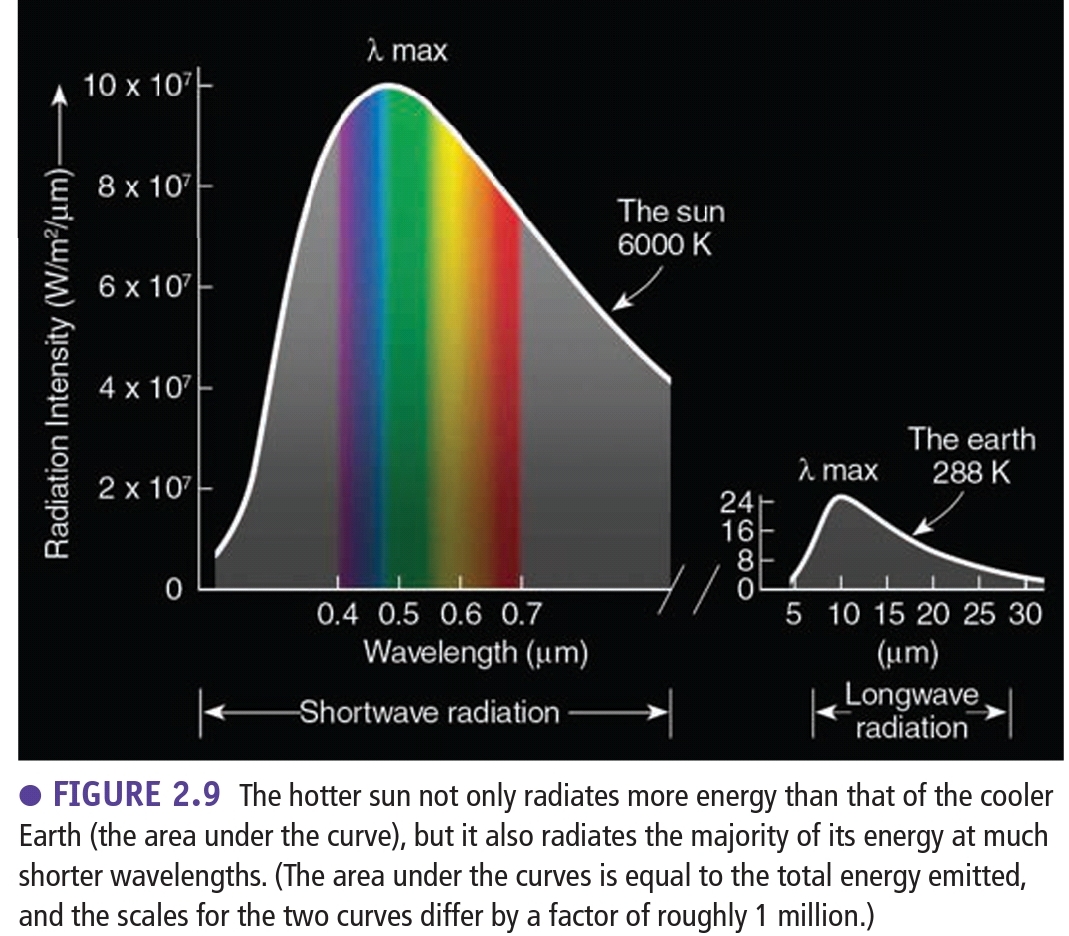
Radiation emitted by the Earth
Earth emits radiation at longer λ than the sun (Emits less Energy)
> 95% of Earth radiation λ = (2.5, 30)μm (IR)
Laws of rediation
All objects emit radiant energy over range of λ (EVERYTHING emits energy), Unless it’s at “absolute 0” if molecules stop
Hotter objects radiate more energy in the form of short λ than cooler objects
Stephan-Boltzman Law: Hotter objects radiate more total E per unit area
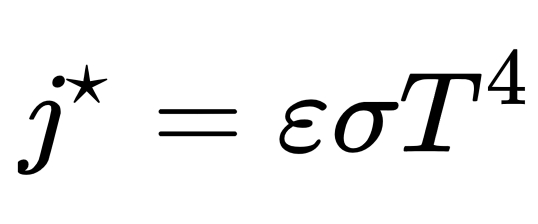
Blackbodies
such as Sun & Earth
Earth’s radiative equilibrium T = 255°k = -18°C = 0°F
Why isn’t this average surface T? atm IS NOT black body (Gases selective absorbers, absorb, & emit IR)

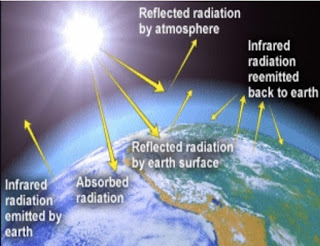

Greenhouse Effect
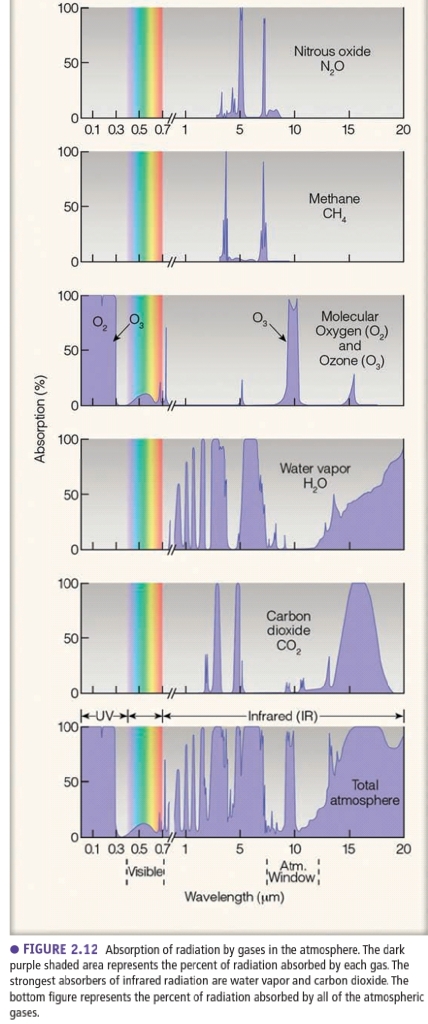
Gases are most effective absorbers of radiation & play primary role in heating atm
1. H₂O, O₂, O₃ absorb most of energy in atm
2. CO₂ is important at long λ (IR)
3. “Openings” are atmospherec windows

Atmosphere warms planet
H₂O, CO₂ (called selective absorber) absorb outgoing IR & absorbed energy heating the air
GHG
The Earth’s average T = 33°C = 59°F
GHG “villain” in Global Warming Debate
GH Effect & Global Warming NOT same thing
Without GH Earth would be uninhabitable!
Human activity may be making atm more efficient at retaining long wave emissions from the Earth
Reflected & Scattered
What Happens to Incoming Radiation?
Absorbed, Transmitted, Reflected, Scattered
DEPENDS ON λ
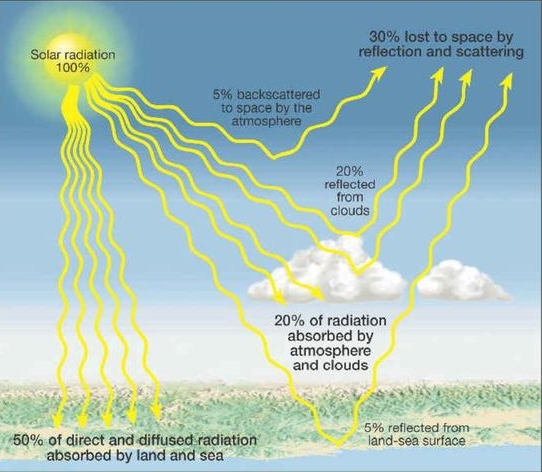
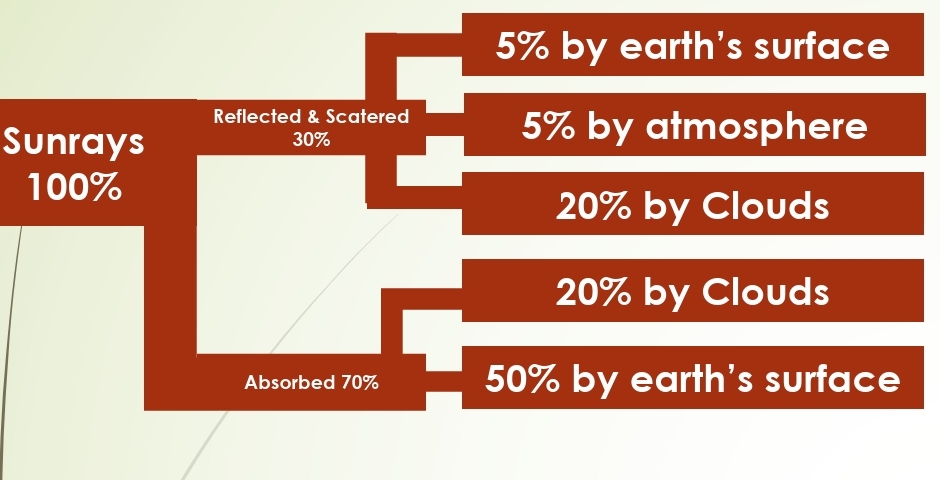
shorter λ (violet, green, blue) are more effectively than longer λ (orange, yellow, red)

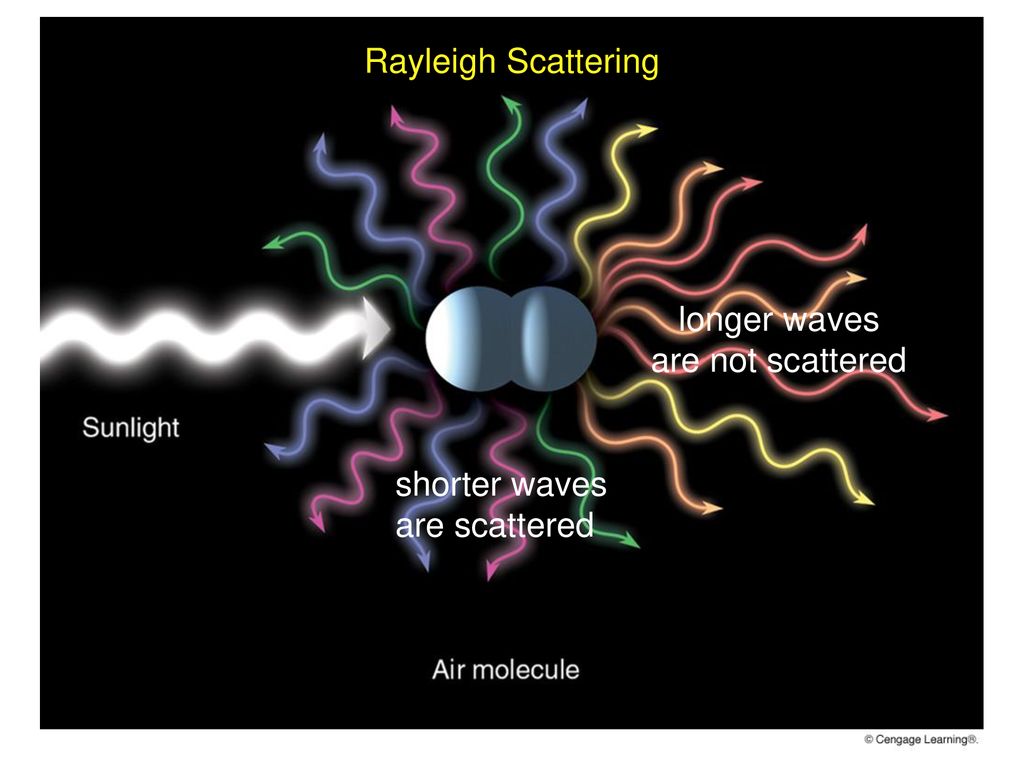
Scattering
produces large number of weaker rays
Air molecules tend to selectively scatter
Blue Skies, Red Suns, & White Clouds duo to scattering
air molecules selectively scatter the SW of VL “green, violet, & blue” more effectively than the LW “red, orange, & yellow” When SW reach our eyes, the brain processes them as blue. So blue light strikes our eyes from all directions, making the sky appear blue
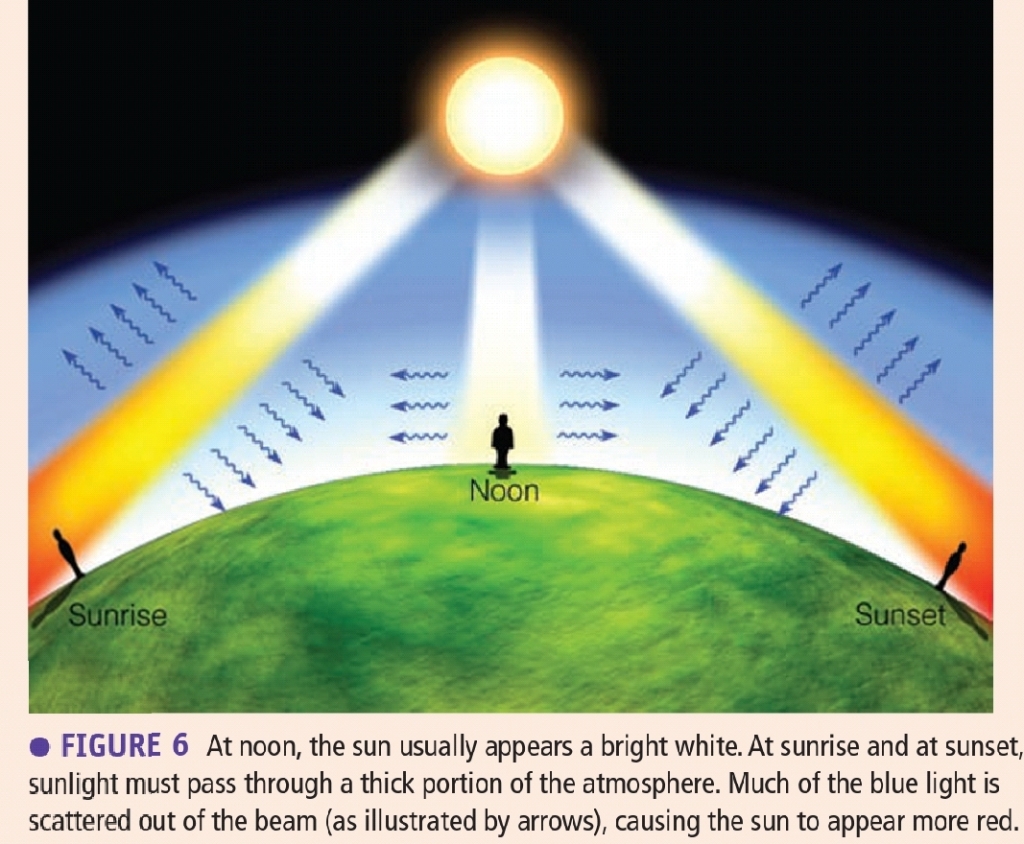
Red Suns
At noon, the sun is perceived as white because all VL strike our eyes, & At sunrise & sunset, the white light pass via a thick portion of atm. Scattering of light by air removes the SW (blue) from the beam, leaving the LW, This situation often creates the image of a ruddy sun at sunrise & sunset
White Clouds
Cloud droplets are much larger than air & don’t selectively scatter sunlight. these larger droplets scatter all VL more or less equally, & appear white due to million of cloud droplets scatter all VL about equally in all directions.
Reflection
bounces off at same angle & intensity
Reflection & Albedo: Energy is returned to space via reflection & emission
ALBEDO: percentage reflected (30%)
5% from land & ocean
25% from clouds & ice

Clouds absorbers IR radiation & effect of heating the earth depends on type of cloud
The effect of heating depends on type of cloud
1. Thick cloud absorbs the most of outgoing IR, & re-radiating it back to the surface (Warm cloudy nights)
2. High Thin Clouds Tend to WARM the surface by transmit incoming SW & Absorb outgoing LW & re-emit it back down
3. Low Thick Clouds Tend to COOL the surface by block incoming SW, have high albedo so reflect SW back to space
On average: clouds Cool the Earth
Note. SW = Short Wave, LW = long wave

T decrease in troposphere, Why
Surface warms the traposphere (The atm is HEATED from the GROUND UP)
Whether specific clouds will warm or cool surface depends on
1. time of day
2. Cloud’s thickness & height above surface
3. Surface with Liquid, dry Land, or ice
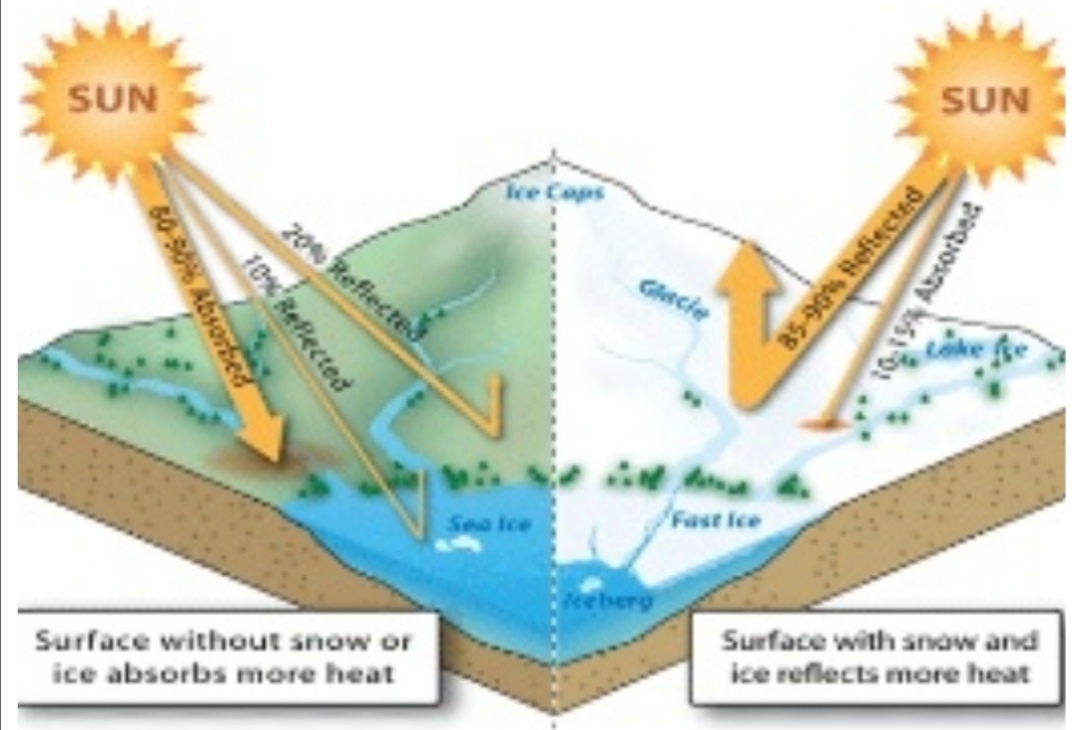
Surface with ice absorb 10-15%, reflect 85-90% of radiation
Earth’s average T constant due to
balance of incoming & outgoing radiation (black body)
Seasons-Regulated by
amount of solar energy received by surface
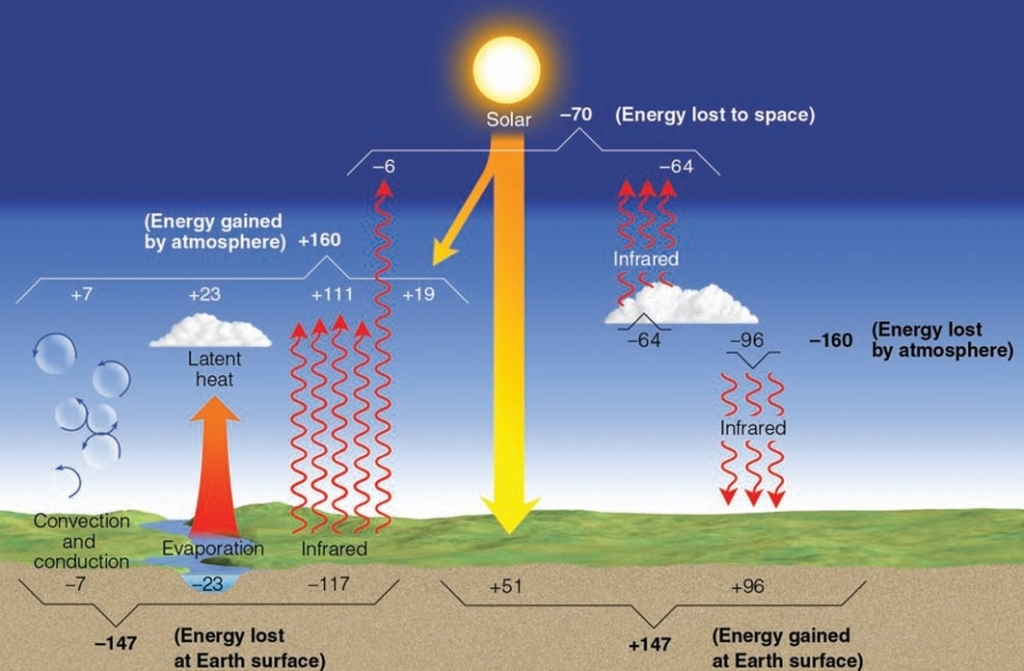
Why don’t tropics keep getting hotter & poles colder?
Movement of atm & oceans transfer energy from the equator to the poles
energy imbalance (unequal heat) drives ocean currents & winds (Weather)
Earth-Sun Relationships
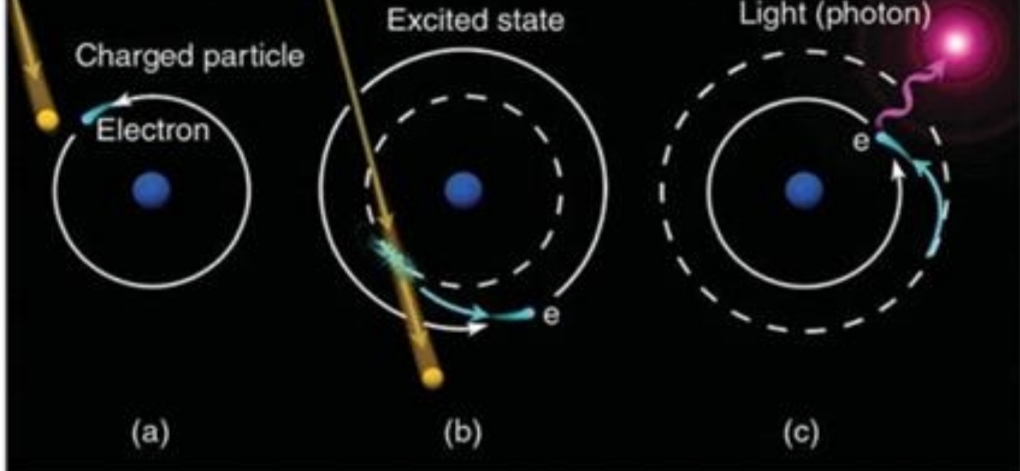
electrons in its normal orbit transfer into higher energy level & When returns to its normal orbit, emits photon
The End
