Online Quiz
The Numerical Dating of the Earth
Dating of the Earth
Early Attempts to Date the earth
Scriptures: the earth was about 6000yr old
Buffon in 1760: the earth was 75,000yr old
based on the uniformity doctrine, Hutton, Lyell thought that the earth must be far older than the above dates
C. Darwin in 1859 needs millions of years before the appearance of human beings based in his evolution theory
In 19th century, a British geologist estimated that all the time since the beginning of the Cambrian ≈ 75 MY, He based his conclusion upon the max. known thickness of strata of that age span multiplied by an assumed average rate of sedimentation (derived from study of modern depositional rates)
In 1899 Joly used the accumulation rate of salt in the sea, he found that it would taken 100MY to develop the present salinity
All the above attempts at assessing the age of the earth in terms of numerical time were based upon shaky assumptions, just all of them showed an evolution of thought in one direction, toward older & older estimates
In middle of 19th century, most geologists thought that the earth date is several hundred MY but they had no numerical method to prove that
Kelvin’s Dating
Deep mines showed that Temperature increase with depth, this leads to one fact that the earth losing heat from its interior
The heat lose could be a result of:
1. It cooling after an initial, very hot stage
2. It contains internal source of heat energy
Lyell appealed to chemical reactions in the interior to produce heat in an endless cycle, allowing for a steady-state earth
In 1846 Kelvin refused Lyell assumption because he reasoned the heat to another assumption that earth originally molten
By using the rate of cooling of the earth backward to a time when the earth was molten, Kelvin thought that the total age of the earth is between 20-30MY
Chamberlin Dating
In 1899 Chamberlin challenged Kelvin assumption that the earth had begun as a molten body
Chamberlin introduced new hypothesis that planets formed by the accretion, or collecting together, of cold, solid chunks mater
So earth must have heated up sometime after its initial formation via some internal process quite independent of solar heat
Summery
Scriptures → 6000yr old
Buffon → 75,000yr old
Hutton, Lyell →>> 75,000yr
C. Darwin → millions of years “evolution”
British geologist →preambrian 75 MY, based on average rate of sedimentation
Joly → 100MY “accumulation rate of salt”
19th, Most geologists → several hundred MY
Kelvin → 20-30MY “cooling rate of the earth”
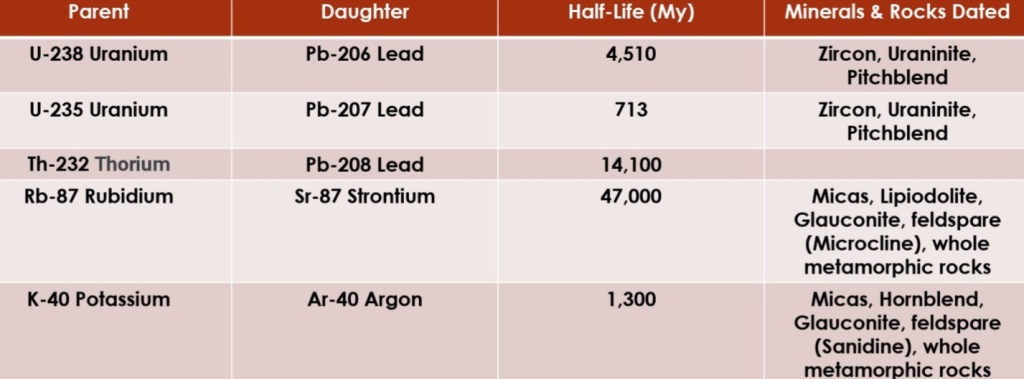
Radioactivity
Important Terms
Radioactivity: elements changing into other by emitting charged particles (Spontaneous changes in structure of atomic nuclei)
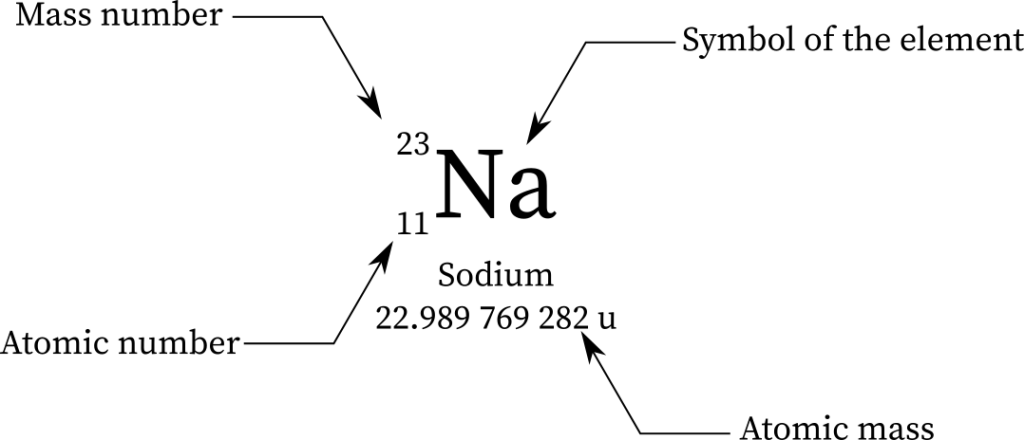
Isotopes: elements have the same atomic number & almost same chemical properties, but atomic weight are different (designated by Mass Number)
Atomic Number: number of proton
Mass Number: number of the total sum of neutrons & protons, written at the top left of the chemical symbol
Parent isotopes: isotopes with unstable nuclei, & undergo spontaneous changes until a permanent, stable configuration is reached
Daughter isotopes: isotopes with stable configuration nuclei
Radioactive decay: process of change Parent to Daughter isotope
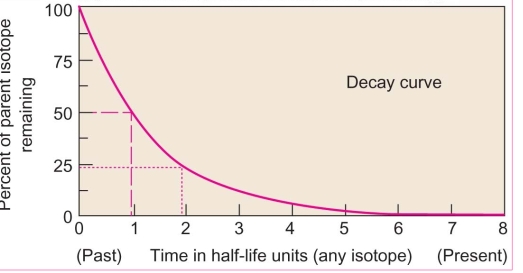
Dating: determining the ratio of parent to daughter atoms & calculating a mineral’s age by multiplying by decay rate of the parent & This leads to half-time units
half-time time required for one-half of the parent isotope in a sample to decay
Radioactivity
discovered by Henri Becquerel & Curies in 1896, by the films story
2 unknown elements were soon discovered (Radium & Polonium) which form from U by changes in the atomic nucleus that involve unknown types of radiation & This lead to the Isotope definition
Radioactive decay
results in one or more of 3 types of Emissions from the nucleus at a fixed average rate characteristic of any isotope
The Emission types
1. Alpha rays: helium nucleus (He+2e-)
2. Beta rays: free electrons (e-)
3. Gamma rays: similar to X rays
The radioactive decay types
1. α-emission: emission α-particle, 2p⁺+2n±
2. β-emission: e- ejected from the nucleus
3. Electron capture: e- captured by nucleus (combines with p⁺ to form n±)
RA-decay can be written like reaction
Unstability Isotope→ Stability Isotopes
Parent → Daughter + (α, β, γ, e) + heat
Emissions result in increasing or decreasing of the number of the neutrons
Ex. U238 decay α particles He, this reduce the neutrons & protons 2 of each, the resulting isotope has amass number 234 (Thorium)
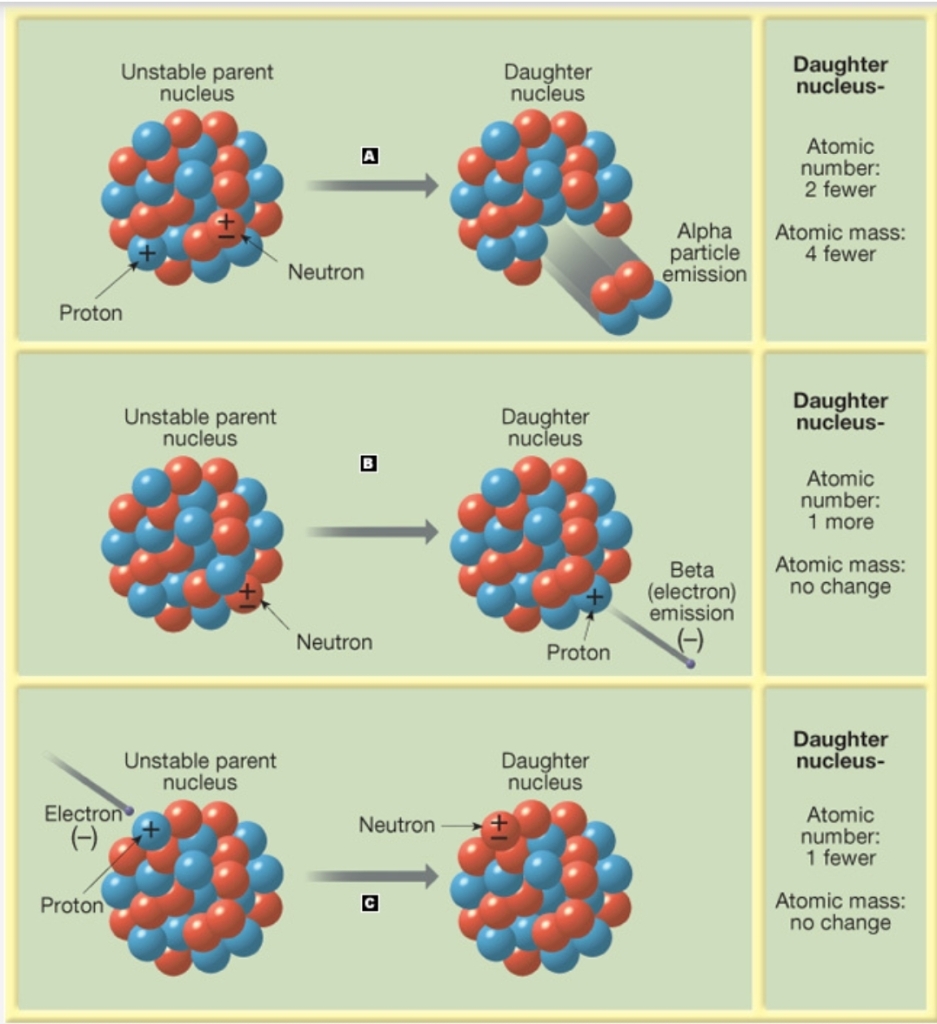
b. β-emission
c. Electron capture
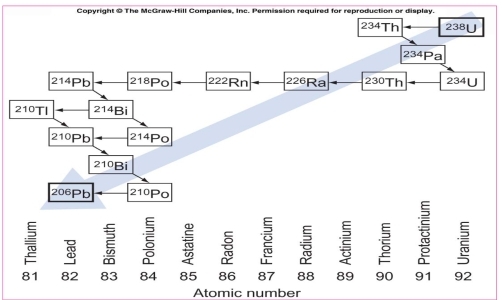
Rates of decay vary from less than 1second (Polonium214) to 1,622yr (radium226)
First Dating of Minerals
In 1902, Rutherford & Soddy stated: total emission activity was proportional to number of unstable parent atoms
Based on this statement:
1. the emission decrease regularly which can be expressed mathematically
2. the progressive accumulation of daughter isotopes might provide basis for the numerical measurement of geologic time

Dating of Minerals
time of the crystallization of a mineral dated by isotopes (not the time of origin of isotope in the universe, which was earlier than origin of the earth), such as the time of incorporation of unstable isotope into that mineral
When mineral crystallized, a closed chemical system was formed such that any daughter product then present in the mineral was formed only from the decay of the original parent isotope therein
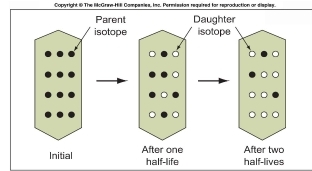
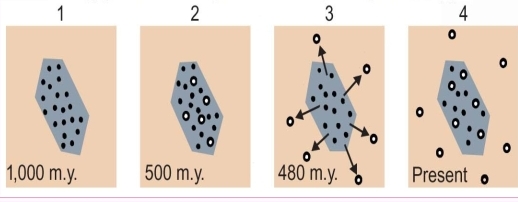
1. crystallized originally 1000MY ago
2. after 500MY, some parent isotopes (dots) in a feldspar crystal had decayed
3. metamorphism 480MY ago drove daughter atoms out of crystals, & they retained in the surrounding rocks
4. present dating of the feldspar would reveal the metamorphic events whereas a total-rock date would reveal original crystallization 1,000MY ago
Accuracy of Isotopic Dates
There is several factors limit the accuracy of isotopic dates:
1. The statistical nature of decay even under the best conditions, only average decay rates are determinable (introduces uncertainty)
2. uncertainty originates in the laboratory analyses of the isotope ratios: mass spectrometer provides the most sensitive analyses of isotope abundance, with from ±0.2 to ±2.0% accuracy possible
Isotopic Time Scale
the bulk of isotopic dating has been confined to igneous or metamorphic rocks, Because most datable minerals containing radioactive elements originate in such rocks
Dating sedimentary rocks by isotopes: date of crystallization of the minerals in parent rocks prior to erosion, transportation & deposition in a sediment
Direct isotopic dates of sedimentary rocks are possible for only a few minerals that:
1. contain unstable species
2. crystallize in depositional environment
– Ex. Gluconite contain K40, so the Mineral can be dated by the K-Ar method
Because most dating must be done on igneous or metamorphic rocks, it necessary to relate such ages indirectly to the relative time scale in order to establish a numerical scale
the basic principles of stratigraphy come into play, especially when:
1. A volcanic rock interstratified within fossiliferous sediments
2. An intrusive igneous body cut or lay the sedimentary strata
Using radioactivity in dating importance of radiometric dating
– Allows us to calibrate geologic timescale
– Determines geologic history
– Confirms idea: geologic time is immense
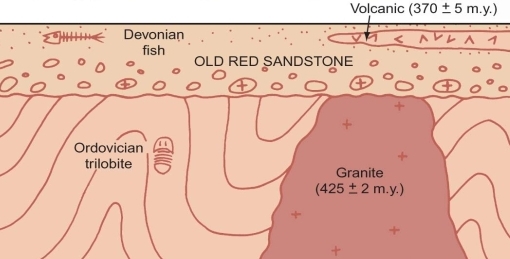
A date for it establishes the Silurian Period as about 425MY old
The date for a lava flow within the old red sandstone establishes that part of the Devonian Period is about 370MY old
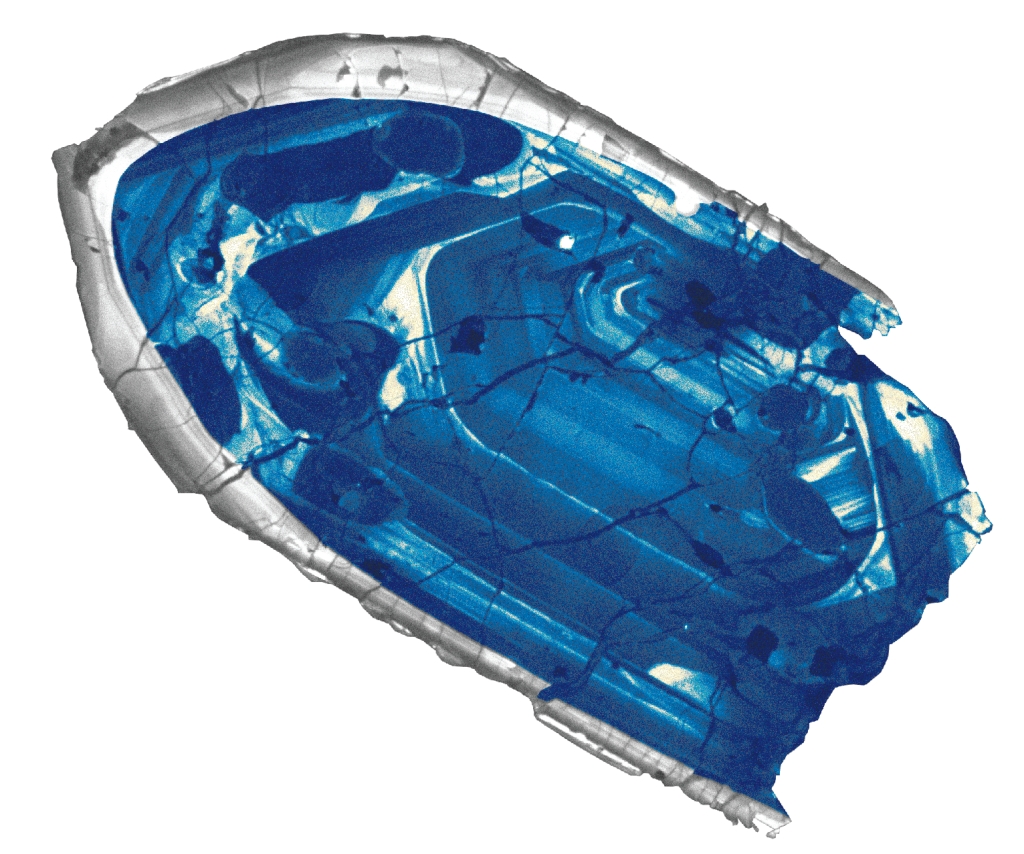
the rock (conglomerate) is 3Ga & contains detrital grains of zircon (mineral formed in granite in crust) that is 4.4Ga
Age of the Earth is 4.54Ga

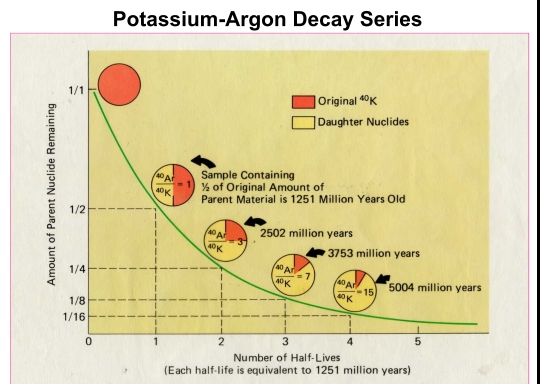
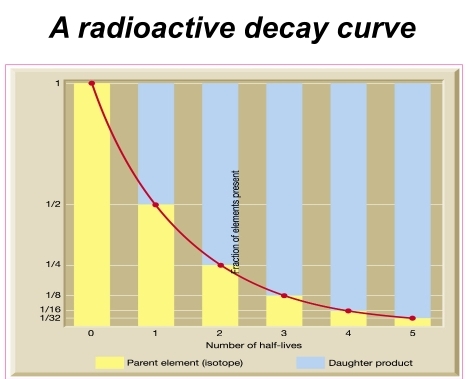
radiocarbon dating
Is the Dating with carbon-14 (¹⁴C)
Half-life of ¹⁴C = 5730yr
Used to date very young rocks
¹⁴C is produced in upper atmosphere
Useful tool for geologists who study very recent Earth history
