Copyright: Foundations of Earth Science, 7th ed
Online Quiz

Minerals
Building Blocks of Rocks
mineralogy: study of minerals
Minerals: Naturally occurring, inorganic, solid with crystalline structure & defined chemical & physical properties that allow it for some variation
Naturally occurring: Form by natural geologic processes, so Synthetic materials are not considered minerals
Inorganic: Crystalline solids from organic sources are not minerals
Some organisms secrete inorganic compounds like calcium carbonate, these material considered a mineral if they become part of the rock record
Solid exception is mercury –> liquid
Crystalline structure Atoms arranged in an organized, & repetitive manner, Organization is reflected in the crystal shape
Chemical composition that allows for some variation: Most minerals are compounds so can be expressed as a chemical formula such as quartz SiO₂
– Composition vary due to substitute for elements & substituting of elements have the same size will not change the crystalline structure of the mineral
Important in human history
1. Flint & chert for weapons & tools
2. Gold, silver, & copper mined by Egyptians
3. Bronze developed by 2200 BC
4. Mining became common by Middle Ages

rocks
naturally occurring solid aggregate mass of mineral, or mineral-like matter
Most aggregates of several different minerals
Individual properties of the minerals are retained
Some rocks are composed of a single mineral, Such as limestone → calcite
Some rocks made of non-mineral, Such as obsidian & pumice (glass), & coal (organic)

Atoms
Building Blocks of Minerals
All matter, including minerals, is composed of atoms, & All atoms (excluding H & He) formed inside massive stars by fusion
Atom: the smallest particle that cannot be chemically split

Atoms contain even smaller particles: Protons, Neutrons, & Electrons
Protons, Neutrons, & Electrons
Protons & neutrons have almost identical masses, & Electrons are much smaller (1/2000) than protons & neutrons
Protons +ve
Found in the nucleus
atomic number: number of P⁺ in the nucleus, & Determines chemical nature of atom
element: atom with the same atomic number
Electrons – ve
Surround the nucleus like a cloud
Valence electrons interact to form bonds
Move around the nucleus in a cloud with different regions called principle shells
Each principle shell has an energy level & a specific number of electrons
The outer shell contains valence electrons
Interact with valence electrons of other atoms to form chemical bonds
Neutrons no charge
Found in the nucleus
Most matter is neutral, because the charges of P⁺ & e- cancel each other out


Elements are arranged in the periodic table
Elements with similar properties line up in columns

2 or more elements joined together, A few minerals are made up of single elements
Native minerals
Atoms Bond
Elements (excluding noble gasses) form bonds under the T & P conditions that occur on Earth
Bonds lower the total energy of the atoms & make them more stable

Atoms tend to gain, lose, or share electrons until they have eight valence electrons
Eight valence electrons is a stable arrangement & a full valence shell
The noble gasses all have full valence shells so they lack chemical reactivity
Elements gain, lose, or share electrons during chemical reactions producing stable electron arrangements
A chemical bond
transfer or sharing of electrons that results in a full valence shell
Ionic bonds: electrons are transferred
Covalent bonds: electrons are shared
Metallic bonds: electrons move around
Ionic Bonds
Electrons Transferred
When one atom loses or gains valence electron, ions are formed
1. Electrons are lost: becomes +ve ion
2. Electrons are gained: becomes – ve ion
Ionic bond form when ions with opposite charges attracted, & Creates ionic compound
Ionic compounds have very different properties than the bonded elements that make them up
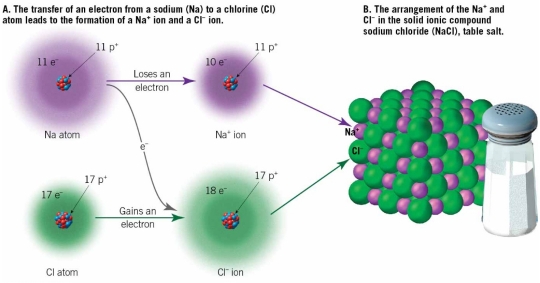
Na loses a valence electron (becomes +ve)
Cl gains a valence electron (becomes – ve)
Both have the same charge
Sodium: Soft, silver, toxic metal that reacts explosively when exposed to water
Chlorine: Poisonous green gas

Metallic Bonds
Electrons Free to Move
form when valence electrons are free to move from one atom to another
All atoms share available valence electrons
Movement of e- between atoms results in:
1. High electrical conductivity
2. Malleability
3. Other unique properties of metals
Physical Properties
Minerals have a definite crystalline structure & chemical composition that Gives them unique physical & chemical properties & These properties can be used in identification
Include
Luster
Ability to transmit light
Color
Streak
Crystal Shape or Habit
Strength (Tenacity, Hardness, Cleavage, Fracture)
Density & Specific Gravity
other distinctive properties (Taste, Feel, Smell, Magnetism, Optical properties, & Effervescence)
Luster
quality of light reflected from the surface of a mineral
Types of Luster
metallic: Minerals that look like shiny metal
submetallic: appears slightly dull
Nonmetallic: Vitreous or glassy, dull, earthy, pearly, silky, & greasy
Ability to transmit light
opaque: do not transmit light are
translucent: transmit light, but not image
transparent: transmit both light & images
Color
may be one of the most obvious properties of a mineral, but it is only a diagnostic property for a few minerals
Slight variations in the chemical composition can change the color dramatically

Streak
is the color of a mineral in powdered form
Obtained by rubbing the sample on an unglazed porcelain tile (streak plate)
Streak, unlike color, is generally consistent
Metallic minerals have a dense, & dark streak
Nonmetallic have a light streak
Not all minerals produce a streak

Crystal shape or habit
is the characteristic shape of individual mineral crystals
Most minerals grow in one common shape, but some have 2 or more shapes


strength
determined by the strength of chemical bonds & determines how minerals break or deform under stress
Include
Tenacity, Hardness, Cleavage, Fracture
Tenacity
resistance to breaking or deforming
Minerals with ionic bonds tend to be brittle, shatter under stress
Minerals with metallic bonds are malleable, deformed into shapes & thin sheets
Sectile minerals cut into thin shavings
Elastic minerals return to its original shape after being bent
Hardness
resistance to abrasion or scratching
measured on a scale of 1 to 10 (Moh’s Scale)
Can be determined by rubbing the mineral against a material of known hardness

Cleavage
tendency of a mineral to break along planes of weak bonding
This produces smooth, & flat surfaces
Not all minerals have cleavage
Cleavage easily confused with crystal shape
cleavage is visible when a mineral is broken


Fracture
property resulting from chemical bonds that are approximately equal in strength
Irregular: uneven broken surface
Conchoidal: smooth, curved broken surface
Some minerals exhibit splintery or fibrous broken surfaces

Density & Specific Gravity
Specific gravity describes the density
Specific gravity G: Ratio of a mineral’s weight to an equal volume of water
Most minerals have G between 2 & 3
Many of the metallic minerals have a much higher G (20 for gold)
G estimated by hefting mineral in your hand
other distinctive properties
Taste halite is salty
Feel talc is soapy, & graphite is greasy
Smell sulfur smells like rotten eggs
Magnetism some can be picked up by a magnet & some can pick up iron objects
Optical properties calcite refracts light
Effervescence carbonate minerals fizz when exposed to dilute acid HCl


Mineral Groups
There are > 4000 named minerals, but only a few are abundant in Earth’s crust known as rock-forming minerals
Economic minerals are less common than rock-forming minerals, but are used in the manufacture of products
silicate groups: Silica & oxygen combined to form the basic building block for the silicates
The most common minerals
More than 800 silicate minerals
Make up 90% of the Earth’s crust
The remaining mineral groups referred to as the nonsilicates
Far less abundant in Earth’s crust
Some are very important economic minerals

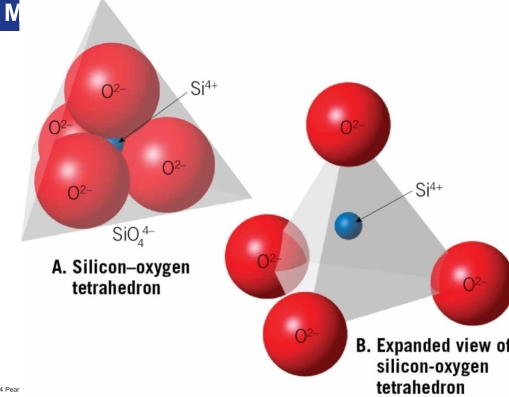
Four O surround Si atom
Tetrahedra can be joined into chains, sheets, or 3D networks by sharing O atoms
Silicate Minerals
Feldspars are the most common silicates, > 50% of Earth’s crust
Quartz is second-most abundant mineral in continental crust
Only common mineral composed completely of Si & O
Silicate minerals tend to cleave between the strong silicon-oxygen structures
Most silicate minerals crystallize from molten rock as it cools
Environment & chemical composition determines which minerals are produced
Some silicate minerals form at Earth’s surface as other silicates are weathered
Some silicate minerals form at extreme pressures during mountain building

Feldspar, Quartz, Muscovite, Clay mineral (Contain Al, K, Ca, & Na)
Dark silicate minerals
iron, magnesium, Pyroxenes, Amphiboles, Olivine, Biotite, Garnet
Dark color & high G from iron content

the most abundant
In Igneous, Sedimentary & Metamorphic
Have 2 directions of cleavage at 90º
hardness = 6
K-feldspar contains K ions
Plagioclase feldspar contains Ca & Na ions, & has striated cleavage surfaces

In Igneous, Sedimentary, & Metamorphic
Impurities cause a variety of colors
hardness = 7
Crystal Forms hexagonal + pyramid

member of the mica family
Excellent cleavage in one direction
hardness = 2.5

the weathering product of other silicates
Common part of soil
Nearly half of the volume of sedimentary rocks is clay minerals

major constituent of dark igneous rocks
Abundant in Earth’s upper mantle
Black to olive green, glassy luster, granular

important in dark-colored igneous rocks
Augite is black, opaque, & has 2 directions of cleavage at nearly 90º
Amphibole
group includes minerals that commonly make up dark portion of light-colored rocks
Hornblende is a dark black mineral with two cleavage planes at 60º and 120º

is a dark, iron-rich member of the mica
Excellent cleavage in one direction
Common in light-colored rocks

is a dark silicate
Glassy luster, no cleavage, conchoidal
Color varies, but commonly deep red

divided into groups based on the – ve charged ion common to the group
Nonsilicates make up only about 8% of crust
Found in significant amount in sedimentary
Some are economically important
Carbonates
contain a carbonate ion CO₃²-
Calcite & dolomite
Used: in road, building stone, & cement
halide
Halite is table salt
sulfate
Gypsum is used in plaster
Halite & gypsum are common evaporites
Oxides
are important iron ores
Other economically important
Sulfides (galena, sphalerite)
Native elements (gold, silver, copper)
Fluorite
Corundum (ruby, sapphire)
Uraninite

Summary
Minerals divided into rock forming menirals that form solid earth & Valuable (economic value), Both may be Silicates or Non-silicates
Silicates (the most common) are based on the
silicon-oxygen tetrahedron, Subdivided into light & dark groups
Nonsilicates include -ve ion, common in sedimentary rocks, & Many ot them are economically important
The End
