Online Quizzes
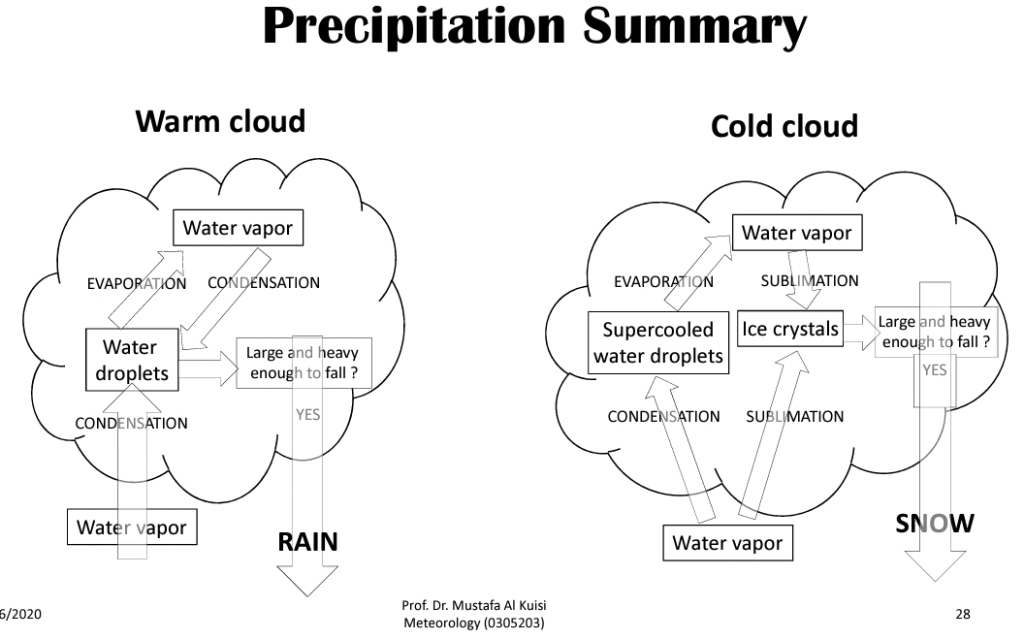
Cloud droplets growth
processes of rain produced
Forms of Precipitation
Precipitation
Cloud droplets growth
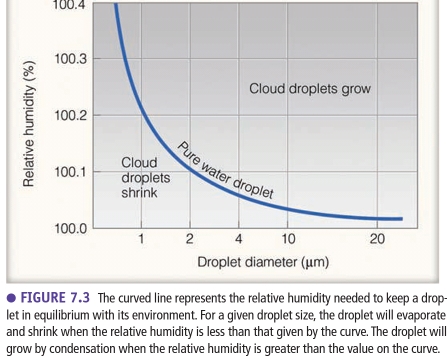
If a cloud droplet is in equilibrium with its surroundings its size doesn’t change because rate of condensing onto the droplet = rate of evaporating from it
If cloud droplet is not in equilibrium its size
– increase if condensation > evaporation
– decrease if condensation < evaporation
To keep the droplet in equilibrium more vapor molecules are needed around it, to replace molecules that evaporating from it
In equilibrium, The total number of vapor molecules around the droplet remains constant & defines the droplet’s saturation vapor pressure
equilibrium vapor pressure is the saturation vapor pressure at equilibrium
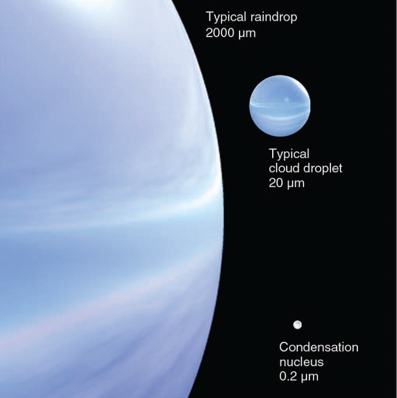
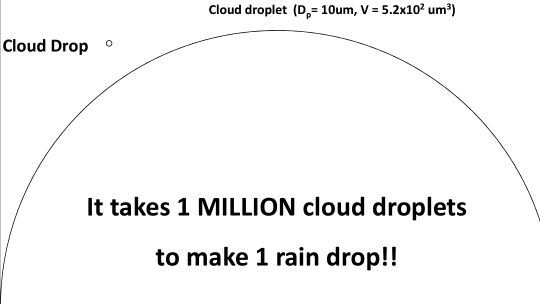
Cloud drops are VERY TINY (0.02 mm)
Rain drops are VERY BIG (2 mm)
curvature effect
When air is saturated with respect to a flat surface, it is unsaturated with respect to a curved droplet (Evaporate)
As a result of curvature effect to keep smaller cloud droplets in equilibrium
→ smaller droplets has greater curvature
→ so more rapid rate of evaporation
→ so require greater vapor pressure
→ The air must be supersaturated (RH>100%)
since the smaller nuclei are more affected by curvature effect, only the larger nuclei are able to become cloud droplets
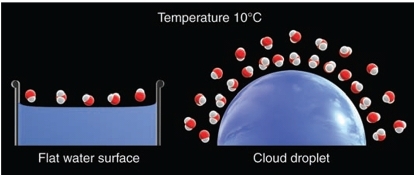
The evaporation over a flat surface of water is less than evaporation of same amount of water over a curved surface
Solute Effect
Condensation begins on tiny particles called cloud condensation nuclei (CCN)
Hygroscopic particles: type of CCN, have an affinity for H₂O, & Condensation begin on it when the RH < 100%, such as salt particles
Salt are dissolve in water forming solution & the salt ions in solution bind closely with H₂O so preventing it from evaporate
solute effect: reduces the equilibrium vapor pressure, by strong association of hygroscopic molecules with water in solution
Due to the solute effect:
1. the salt replaces H₂O in lattice structure
2. the equilibrium vapor pressure is lowered
droplet containing salt can be in equilibrium when the RH << 100%
Should RH increase:
→ water vapor molecules would attach to the droplet at a faster rate than they would leave
→the droplet would grow larger in size
Condensation in Unsaturated air
In moist but unsaturated air:
– condensation occurs as air cools, RH ≈78
– As the air cools further, RH increases, with the droplets containing the most salt reaching the largest sizes.
Over land masses where large concentrations of nuclei exist, several hundreds/cm³, all competing for the available supply of H₂O(g)
Over the oceans where the concentration of nuclei is less, < 100/cm³, but larger cloud droplets
So, in a given volume we tend to find
1. more cloud droplets in clouds over land
2. larger cloud droplets over the ocean
processes of rain produced
Collision‐Coalescence process
Bergeron: the ice‐crystal process
COLLISION & COALESCENCE PROCESS
Air retards the falling drops
The amount of air resistance depends on:
1. rate of fall
2. The size: larger fall faster!
3. Speed: mire speed → more air encounters
4. Coalescence: Large droplets collide & merging with smaller drops in their path
Terminal Velocity: constant speed of falling drop if the air resistance =F.gravity
– The speed of the falling drop increases until the air resistance equals the pull of gravity
larger drops fall faster than smaller drop larger drops have a smaller surface area to weight ratio, they fall faster before reaching their terminal velocity
Coalescence enhanced if colliding droplets have opposite & attractive electrical charges
Rising air currents in a forming cloud, slow the rate of fall toward the ground
amount of time the droplet spends in the cloud An important factor influencing cloud droplet growth by the collision process
Thick cloud with strong updrafts maximizes
1. the time cloud droplets spend in the cloud
2. the size to which they can grow

In a cloud composed of droplets of varying sizes, larger droplets fall faster than smaller droplets. Although some tiny droplets are swept aside, some collect on the larger forward edge, while others (captured in the wake of the larger droplet) coalesce on the droplet’s back side
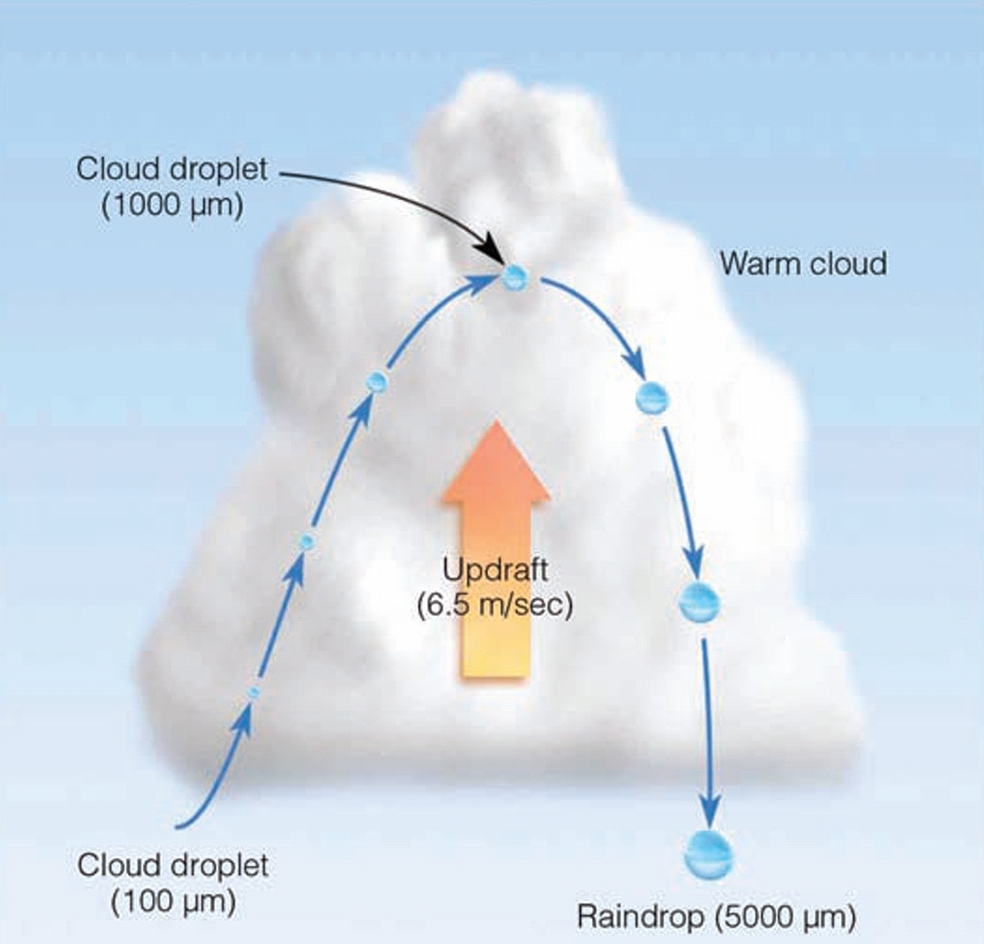
The most important factor in the production of raindrops is the cloud’s liquid water content.
In a cloud with sufficient water, other significant factors are:
1. the range of droplet sizes
2. the cloud thickness
3. the updrafts of the cloud
4. the electric charge of the droplets and the electric field in the cloud
Bergeron Process
process of ice crystal growth that occurs in mixed phase clouds (containing a mixture of supercooled water and ice) in regions where the ambient vapor pressure falls between the saturation vapor pressure over water and the lower saturation vapor pressure over ice.
This is a subsaturated environment for liquid water but a supersaturated environment for ice resulting in rapid evaporation of liquid water and rapid ice crystal growth through vapor deposition
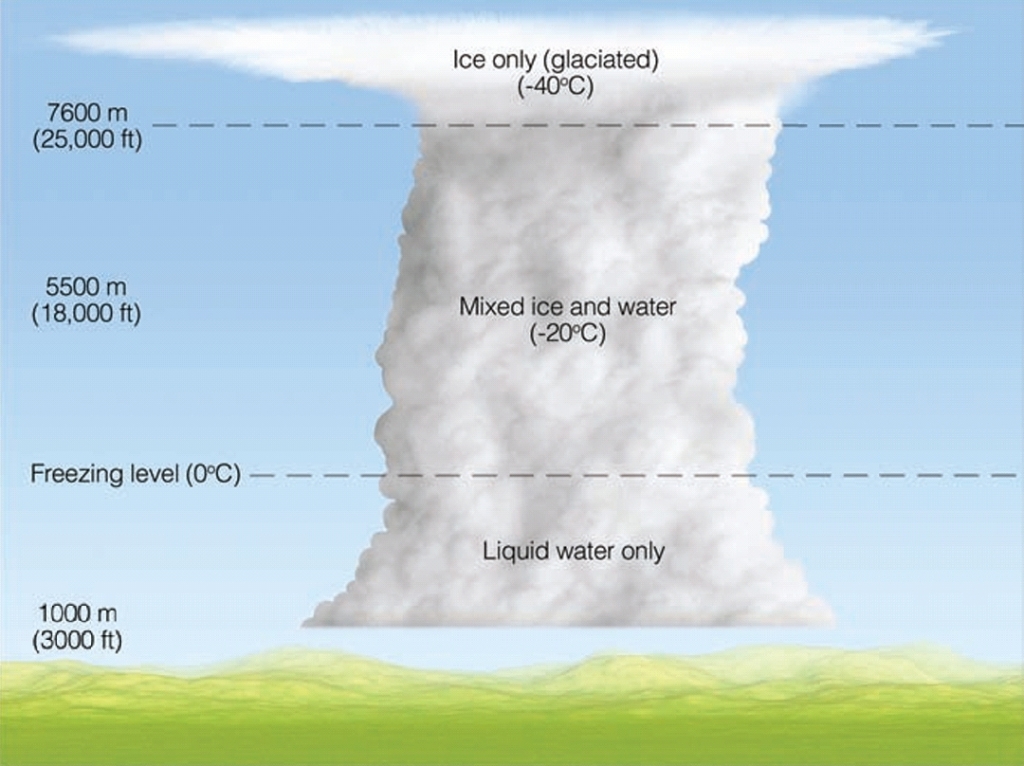
Precipitation in Cold Clouds
Ice, water & water vapor exist at the same time
Cloud drops DON’T FREEZE at 0°C
Liquid water won’t freeze until ‐40C°, SUPERCOOLED
Supercooled water freezes when it touches a Freezing Nuclei, FN are rare
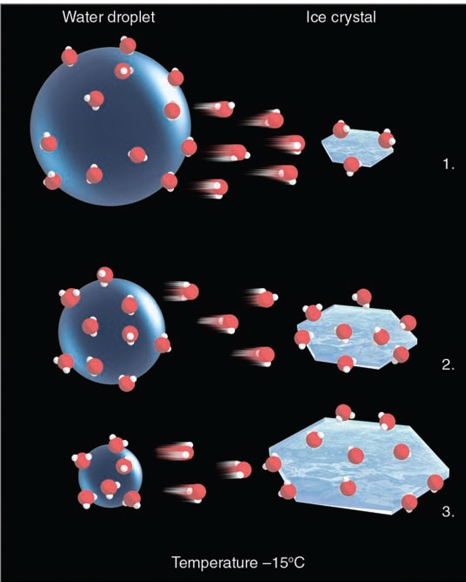
ICE-CRYSTAL PROCESS
The ice‐crystal (or Bergeron) process of rain formation is extremely important in middle and high latitudes, where clouds extend upward into regions where the air temperature is well below freezing.
Such clouds are called cold clouds.
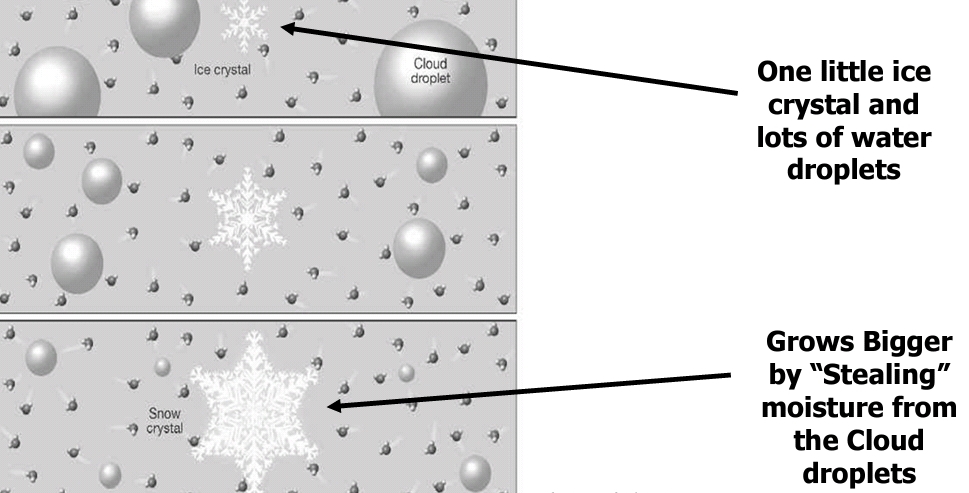
The Bergeron Process often results in liquid precipitation.
As the crystals grow and fall, they pass through the base of the cloud, which may be above freezing.
This causes the crystals to melt and fall as rain.
The Bergeron Process
Summary
The air reaches saturation and some of the resulting droplets will come in contact with freezing nuclei (assuming they have reached the activation temperature).
There is then a combination of ice crystals and supercooled water droplets.
From the perspective of the supercooled droplets, the air is in equilibrium at saturation, but from the perspective of the ice crystals, the air is supersaturated.
water vapor will sublimate on the ice crystals. Since the amount of water vapor in the air has decreased, and from the perspective of the supercooled water droplet, the air is subsaturated, the supercooled water will evaporate until the air once again reaches saturation.
The process then continues.
In short summary, the ice crystal grows through sublimation at the expense of the supercooled water droplet.
Precipitation in Warm Clouds
In warm clouds there are no Ice Crystals so the Bergeron Process can’t operate
Collision‐Coalescence a.k.a. Bump & Stick
Need one BIGGER than average Cloud Drop
Really large aerosol (CCN) → Start Big…. End Big
Entrainment Mixing (evaporation & redistribution)
Turbulence (smashing together of droplets)
Collision-Coalescence
Terminal Velocity matters
the highest velocity attainable by an object as it falls through the air
Sum of the drag force and buoyancyequals the downward force of gravity
If cloud drops were all the same size could they bump into each other? NO
BIG drops fall FASTER than SMALL drops!!!

Cloud Seeding & Precipitation
The primary goal in many cloud seeding experiments is to inject (or seed) a cloud with small particles that will act as nuclei, so that the cloud particles will grow large enough to fall to the surface as precipitation.
Some of the first experiments in cloud seeding were conducted by Vincent Schaefer & Irving Langmuir during the late 1940s.
To seed a cloud, they dropped crushed pellets of dry ice (solid carbon dioxide) from a plane. Because dry ice has a temperature of ‐78°C (‐108°F), it acts as a cooling agent.
“Fake Ice” to simulate the Bergeron Process (Dry Ice, Silver Iodide)
In 1947, Bernard Vonnegut demonstrated that silver iodide (AgI) could be used as a cloud‐ seeding agent.
Because silver iodide has a crystalline structure similar to an ice crystal, it acts as an effective ice nucleus at temperatures of ‐4°C (25°F) & lower.
Silver iodide causes ice crystals to form in two primary ways:
1. Ice crystals form when silver iodide crystals come in contact with supercooled liquid droplets.
2. Ice crystals grow in size as water vapor deposits onto the silver iodide crystal.
Cloud Seeding & Precipitation
Hard to “prove” that it actually worked.
Need the right ratio of cloud droplets to ice crystals.
Concern over toxicity of silver iodide.
You can “overseed” a cloud and too many ice crystals are formed so it doesn’t rain
You can also “overseed” cold fog with dry ice to dissipate it.
Forms of Precipitation
Snow & Hail is seen on Big Island
Sleet, Glaze, & Rime do not seen on Big Island
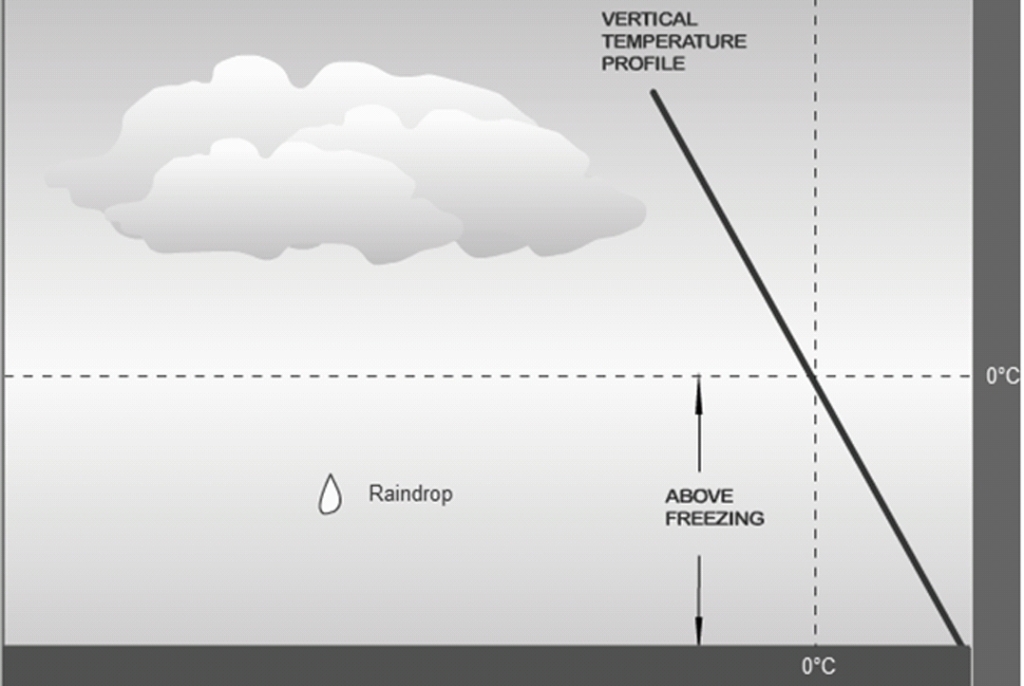
Rain
Drops of water that fall from a cloud & have a diameter of at least 0.5mm
atm is warm, above freezing, from the surface to the cloud layer (Hits the surface as a liquid)
Rain can start out as an ice crystal (Bergeron) or as a large droplet (Collision‐ Coalescence)
DRIZZLE & MIST: Small drops
Virga: Rain that evaporates before reaching the surface
Flooding, Flash Flood
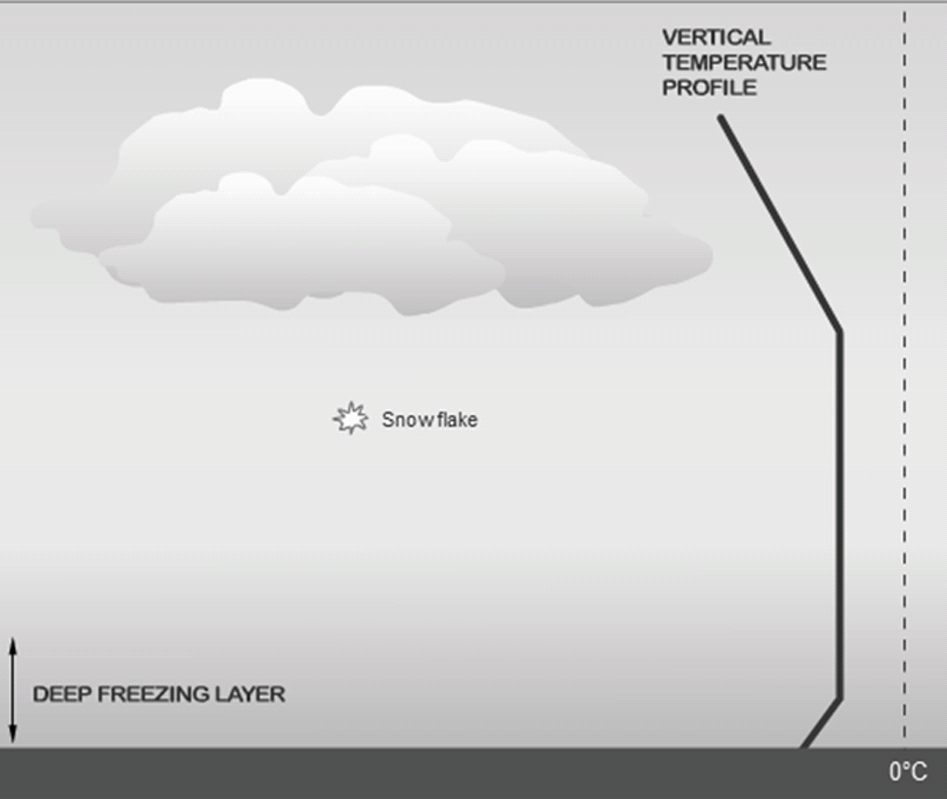
Snow
Ice crystals & clumps
atm is cold, below freezing, from the surface to the cloud layer (Hits the surface as solid ice)
Snow will start out as ice crystal (Bergeron)
Very Cold Conditions
– light snow: Individual crystals
Warmer Cold Conditions
– wet snow: Crystals form clumps
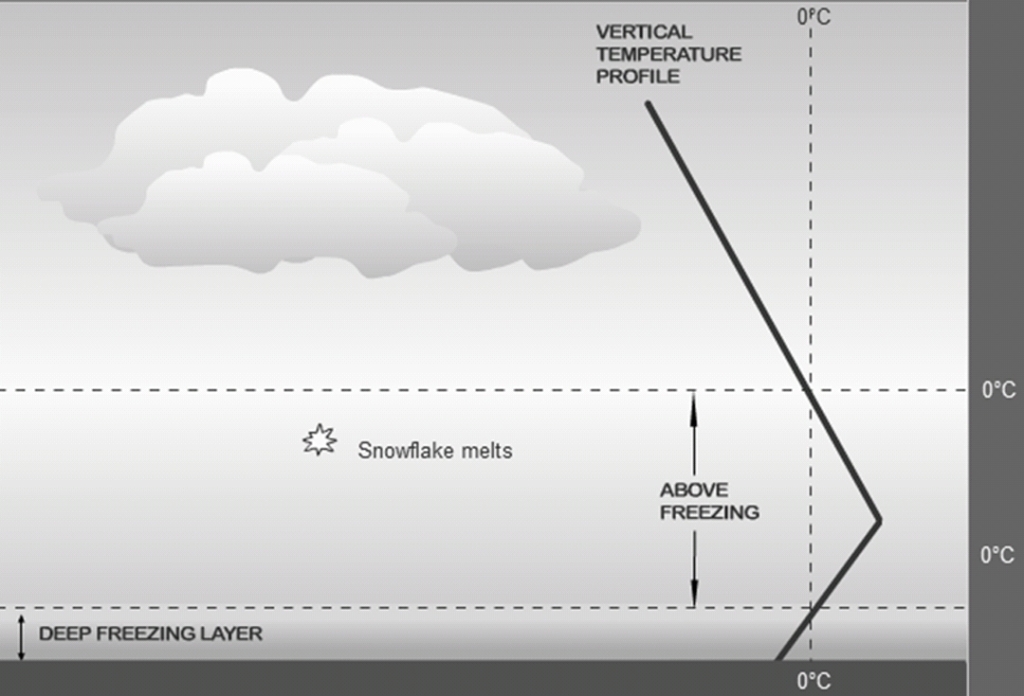
Sleet
Rain that freezes near the surface, Wintertime phenomenon
atm is cold, below freezing, in the cloud layer & at the surface, & there is a layer in between that is above freezing
(Hits the surface as solid ice pellets)
Precipitation (from snow) → rain → sleet
Clearto translucent pellets (Ice Pellets)

Freezing Rain & Glaze
Rain falls in liquid & then freezes at ground
atm is cold (below freezing) in the cloud, above the surface there is a warm (above freezing) layer than melts the snow.
just at the surface there is a layer of below freezing that causes the rain drop to freeze on contact with the surface
Glaze The coating of ice
Hail
Rounded WHITISH Pellets & Irregular lumps of ice, 1‐5 cm & Can weigh > pound
Can be very destructive & damage cars, crops, & even kill people!
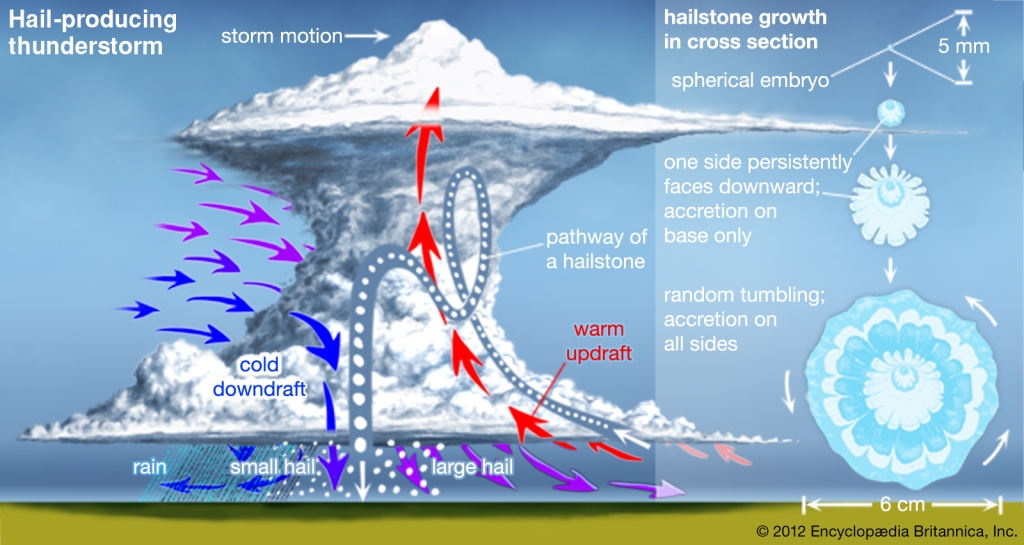
Produced in a Cumulonimbus cloud
Graupleor large frozen rain dropsact as embryos
They accumulate supercooled water, adding new layer
Violent, upsurging air currents within the storm carry these embryos up via the cloud
Low liquid water makes a white layer
Higher liquid water makes a clear layer
When the updraft can no longer keep it aloftit falls to the surface
The more violent the storm the larger the hail can become.
Rime
This is a deposit of ice crystals
Formed by freezing of supercooled fog or cloud droplets on objects whose surface is below freezing
The End
