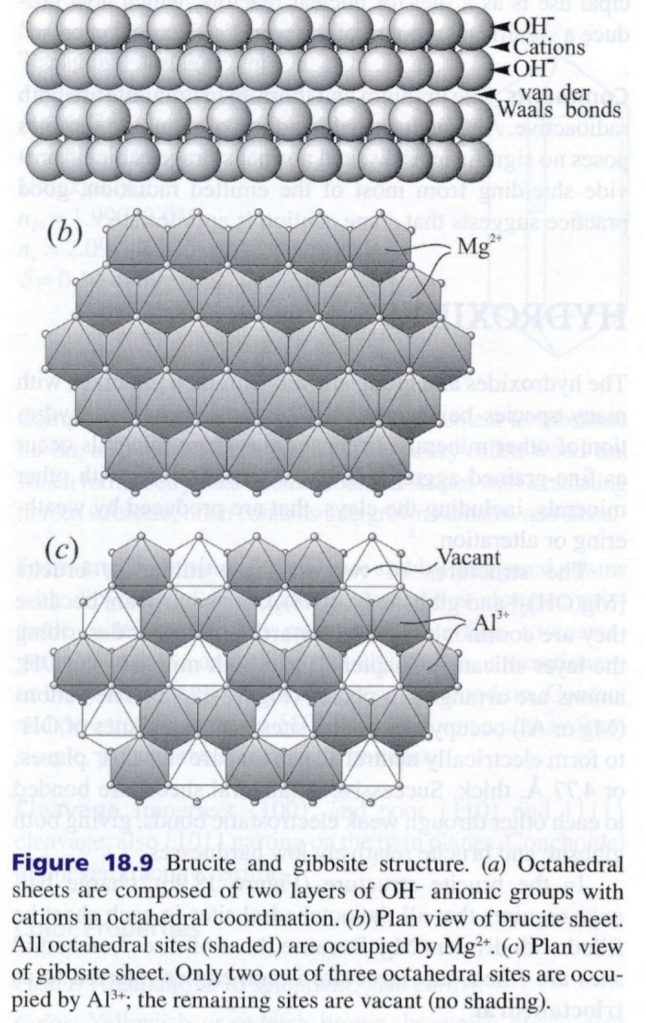
Part.2 : Hydroxides
Brucite Mg(OH)₂
Composition
Fe²⁺ & limited Mn²⁺ may substitute for Mg
Occurrence
– In marble as alteration product after percales
– In serpentinite & chlorite schist, as small veins with talc, magnesite, & other Mg-bearing
Use In refractory materials
Crystal system Hexagonal (Trigonal)
Iron-Hydroxide (limonite)
Limonite: mixtures of Fe-(oxide & hydroxide)
Goethite: αFeO(OH)
– Most common of these minerals
limonite product by withering, hydrothermal alteration by break down of biotite, amphepal, pyroxine, magnitite, & iron-bearing minerals
Large masses of limonite produced by alteration & oxidation of pyrite FeS in hydrothermal sulfide deposits
Intense weathering of Fe-bearing rock result in formation of lateritic soil rich in Fe-hydroxide
Goethite are biomineral: found teeth of chitins, Microbial activity is almost certainly involved in their common
Aluminum Hydroxide (Bauxite)
Bauxite: mixed Al-hydroxide minerals
Bauxite produced in areas subjected to intense weathering, usually in tropical or semitropical climates, & contains clay (kaolin) & Fe-hydroxides
common minerals
gibbsite Al(OH)₃
diaspore αΑlO(OH)
bohmite γΑlO(OH)
found in deposits as withring or altration product of corundom, Al-silicates, & other Al-rich mineral
Manganese Oxide & Hydroxide (Wad)
Wad: materials that include substantial amounts of manganese oxide & hydroxide
Occurrence:
Hydrothermal Systems
Weathering & alteration of Mn bearing & other that contain Mn substituting for Fe-Mg
Some Mn deposits biologically mediated by Mn-oxidizing bacteria
Dendrites (precipitation by groundwater)
Most common minerals
Manganite MnO(OH)
Pyrolusite MnO₂