anionic groups
minerals described in this chapter have structures based on anionic groups that have [-2, -5] charge
Carbonates CO₃²-
Sulfates SO₄²-
Phosphates PO₄³-
Tungstates WO₄²-
Molybdates MoO₄²-
Borates BO₃³-
Part.1 : Carbonates
CARBONATES CO₃²-
OH- groups or other anionic components may be present
Common carbonate minerals divided into:
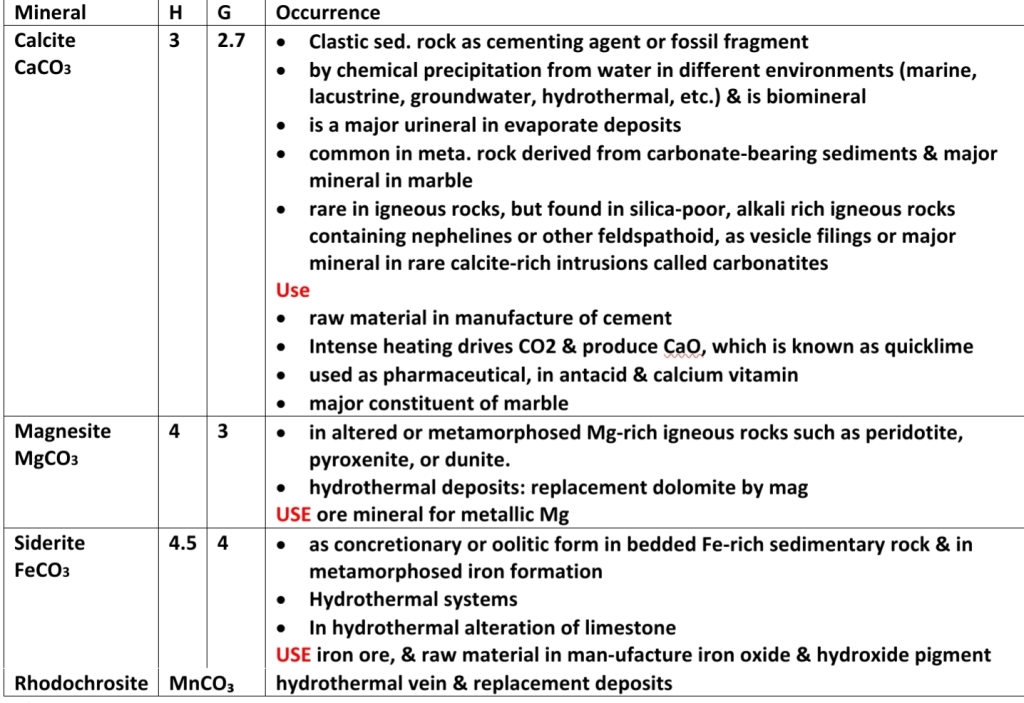
1. Calcite Group: calcite, magnesite, siderite, rhodochrosite, & smithsonite
2. Dolomite: dolomite, ankerit, kutnohorit
3. Aragonite Group: aragonite, witherite, strontianite, & cerussite
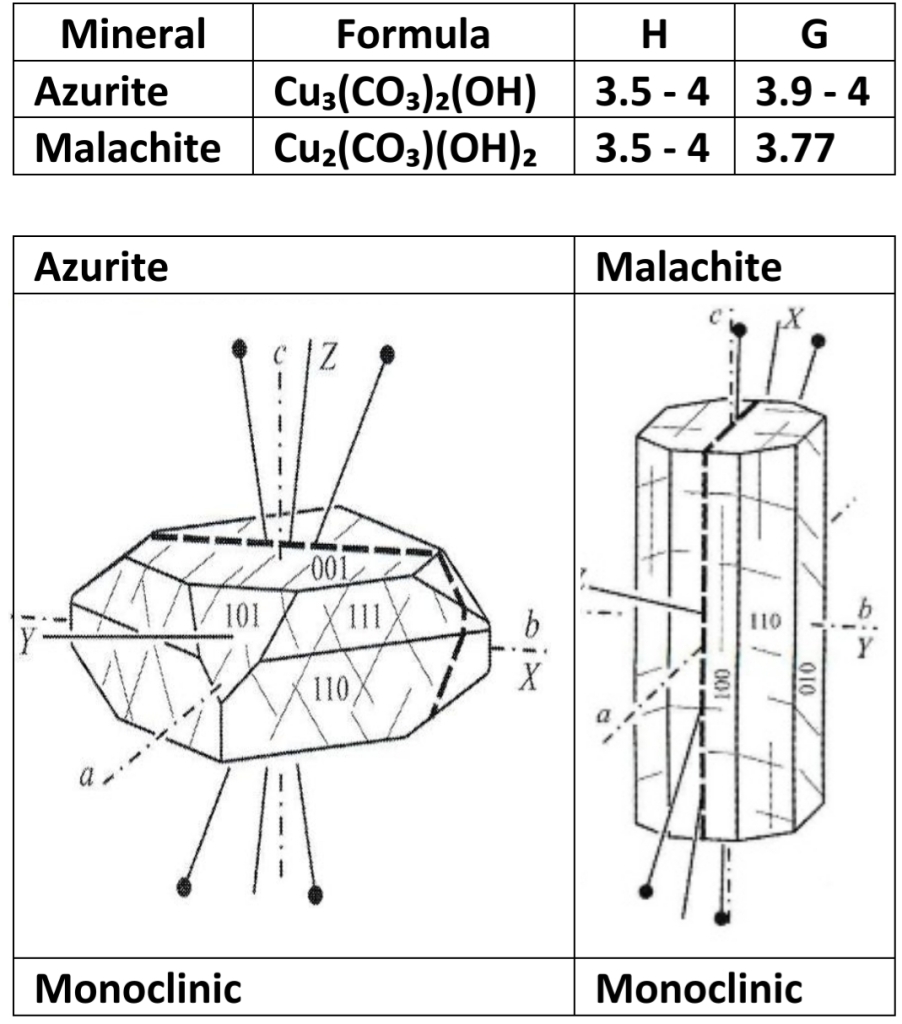
4. OH-Bearing Group: azurite, malachite
RHOMBOHEDRAL CARBONATES
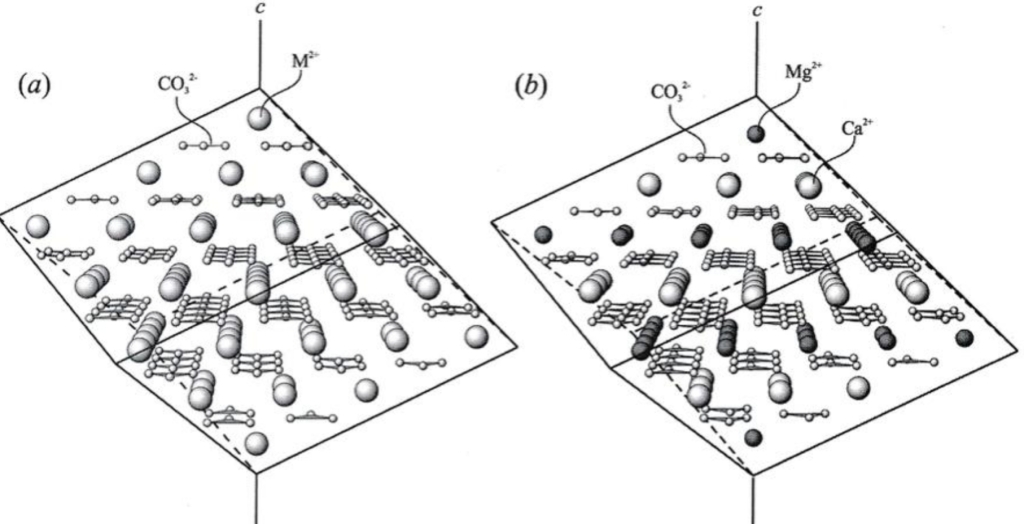
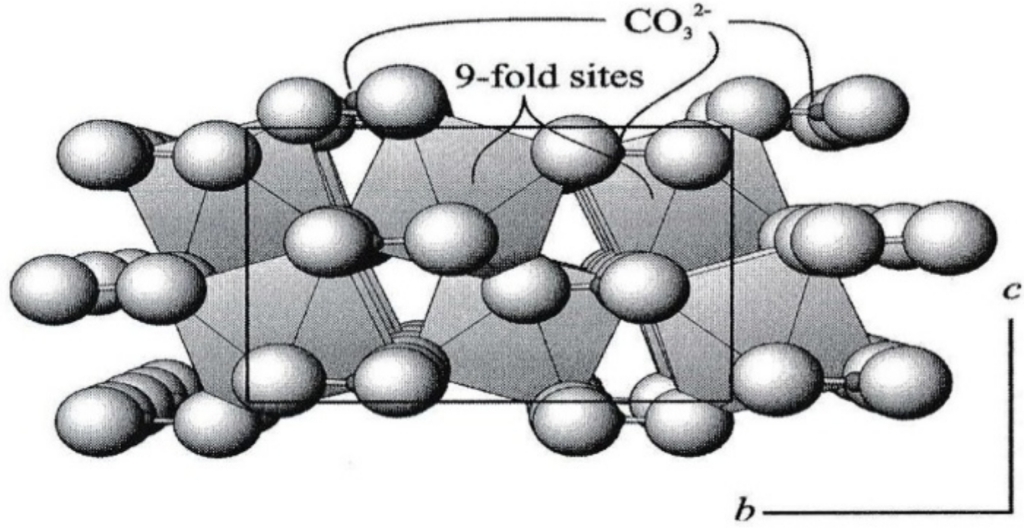
calcite & dolomite groups share same structure & collectively called rhombohedral carbonates, because they have rhombohedral symmetry
Successive layers offset so that position of C is equivalent to cubic close packing, & repeated in every third CO₃ layer, comers of triangular CO₃ altemate by mirror plane every layer (ABC) result is exact duplicate layer of CO₃ groups is repeated only every sixth layer.
Divalent cations (Ca, Mg, Mn, & Fe) occupy octahedral (6-fold) sites, Each O coordinates with one C within its layer, one divalent cation above it, & one below
surfaces of 3 cleavage “rhomb” parallel to 3 edges of triangular carbonate groups
This discussion leads to a problem with nomenclature in rhombohedral carbonates
Solid Solutions
In calcite group: controlled by ionic radii
– between magnesite Mg, & siderite Fe, & between siderite & rhodochrosite is complete
– limited between rhodochrosite & magnesite
– Limited between Ca (1.14) & Mg, Fe, Mn
ionic radii (Mg 0.86), (Fe 0.92), (Ca 1.14), (Mn 0.96)
In dolomite group: Dolomite CaMg(CO₃), & ankerite Ca(Mg,Fe)(CO₃), form solid solution series, & some Mn may substitute for Fe or Mg
orthorhombic aragonite structure favored for cations whose radius in octahedral (> 1.1A) & calcite structure favored for smaller cations (Because Ca²⁺ anionic radius = 1.14 A in octahedral) so fit in either structure
Calcite (Mineral)
Composition:
– nearly pure CaCO₃
– because Crystallization at elevated T allows for some Mg, Fe, Mn, or Zn
– Mg is common substation
– Some larger cations (Ba or Sr) may present
Form
– prismitic, Rhombohedral, scalenohedron
Iceland spar: High-quality clear calcite


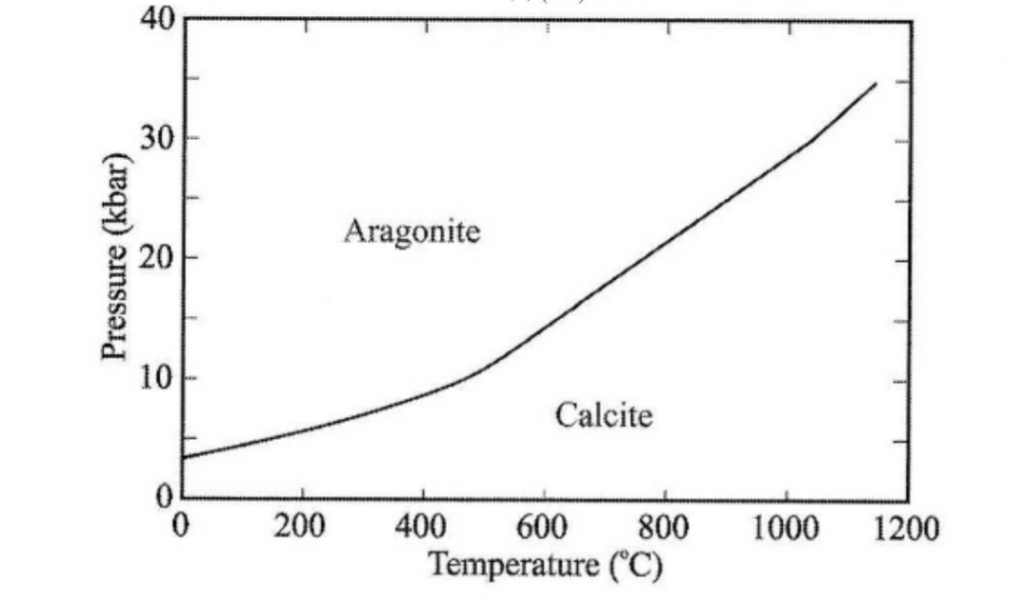
commonly inverts to its polymorph calcite, & pseudomorphs of calcite after aragonite are common, also may be replaced by dolomite

OH-BEARING CARBONATES
Restricted to oxidized portion of Cu-bearing hydrothermal sulfide mineral deposit
Occurrence
1. produced when primary Cu sulfide, such as chalcopyrite, oxidized & altered by acidic water percolating down from surface
2. carbonate component acquired from CO₂, dissolved in meteoric water or from carbonates in host rock for mineral deposit
Use minor ore of copper