Humidity, Condensation, Clouds
Online Quiz

Special Properties of water
– easily changes from solid to liquid to gas
– Ice is LESS dense than water so it floats (Ice cubes, ice bergs, & ice caps)
– Has an unusually high heat capacity (3 times that of land)
– The angle in the water molecule = 104°
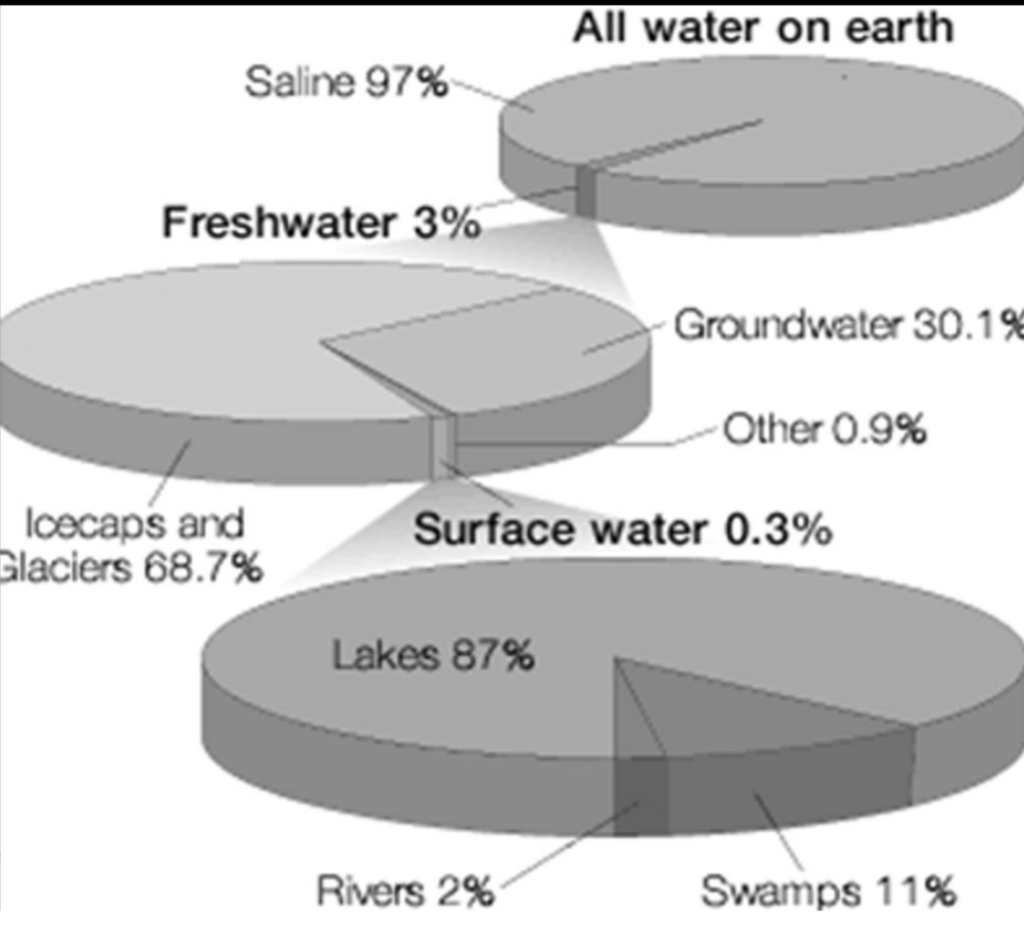
body of the earth is made up of 70% H₂O
Oceans account for most of water (>97%)
Ice sheets in Antarctica & Greenland (< 3%)
Atmosphere has only a little (0.001%)
The water (hydrologic) cycle
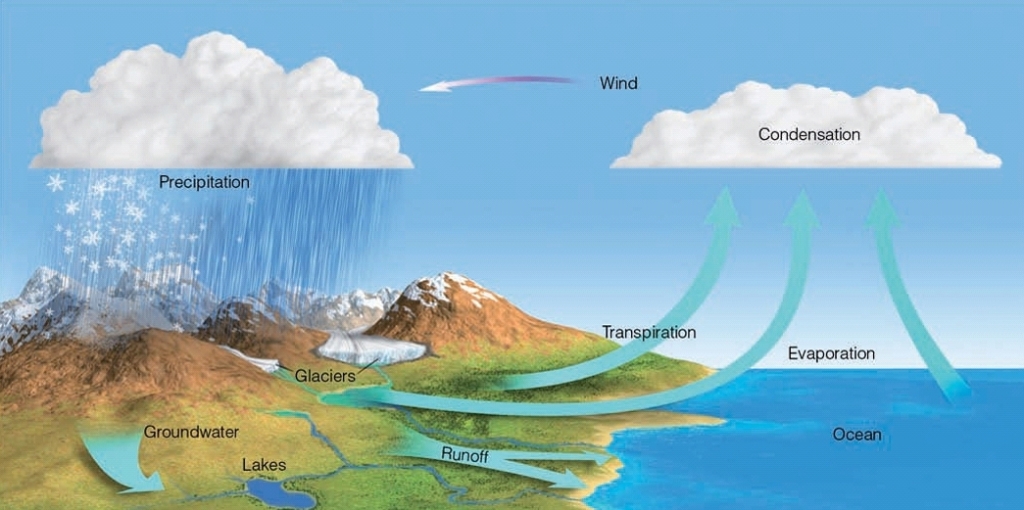
EVAPORATION (Require Energy)
Process by which liquid transformed into gas
Happens over Oceans, Lakes, Rivers & other “standing” bodies of water
Powered by the sun: Solar radiation heats up water molecules until they are “freed” from the liquid state
CONDENSATION (Release Energy)
The change from a gas to a liquid
Responsible for the formation of clouds
PRECIPITATION
Falling liquid or solid in the atmosphere
Balances Evaporation: Average annual precipitation equals evaporation
Happens over Land or Oceans
Returns the water to the ocean or soaks into the ground
TRANSPIRATION
release of H₂O(g) to atmosphere by plants
Plants uptake water through their roots that fell as precipitation
Not as important as evaporation
SUBLIMATION (Require Energy)
Conversion of a solid directly to a gas
EX: Gradual shrinking of unused ice cubes, the rapid conversion of dry ice into gas.
How piles of snow tend to disappear even if air T never reaches above 32F (if air is dry)
DEPOSITION (Release Energy)
Conversion of a gas directly to a solid, without passing through an intermediate liquid phase
EX: Frost on a window pane (white frost, hoar frost… FROST)
EX: Frost the builds up in the freezer.. Was once part of your ice cube!
Location of Processes
Evaporation exceeds Precipitation over Water (No plants), so no transpiration
Precipitation exceeds Evaporation over Land
Condensation happens everywhere
Water Vapor Content of Air
Humidity
The general term used to describe the amount of H₂O(g) in the air

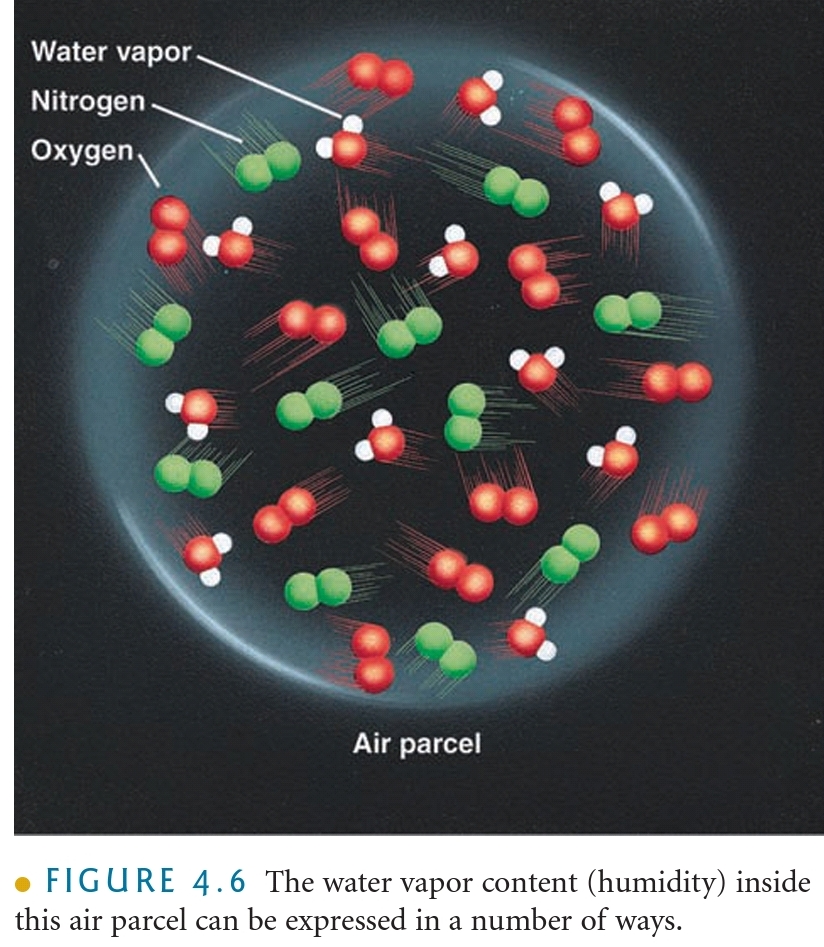
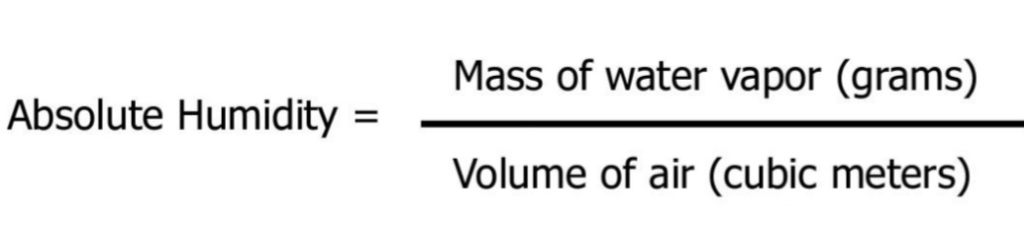
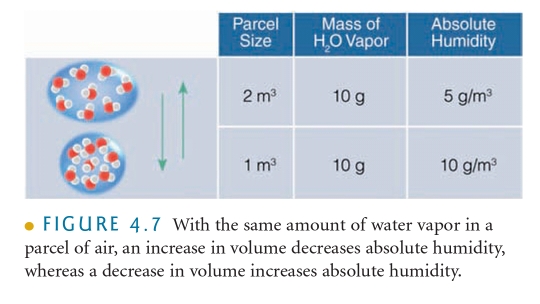
The MASS of water vapor in VOLUME of air
Changes in air P & T change V of air even if the amount of water doesn’t change
AH = density of water, (m/V) Chemistry 101

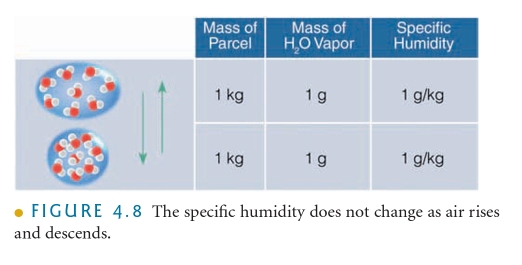
The MASS of water vapor in a unit of air compared to the remaining MASS of air (include water vapor) molecules
SH = percent by mass & Mass fraction, (w/w)% Chemistry 102
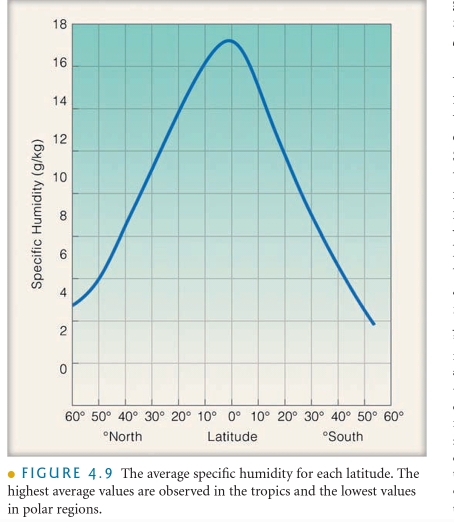
Warm, & Muggy tropics→highest Average SH
Away from the tropics → decreases
the polar latitudes → lowest Average SH
Majority deserts are located near latitude 30° (the average air contains nearly twice the water vapor as does the air at latitude 50°N)
the air of a desert certainly not “dry” nor is the water vapor content extremely low
Since the hot, desert air of the Sahara often contains more water vapor than the cold, polar farther north, summertime Sahara air has a higher specific humidity due to tempature (dew point) The air in the desert can’t carry large amounts of water.
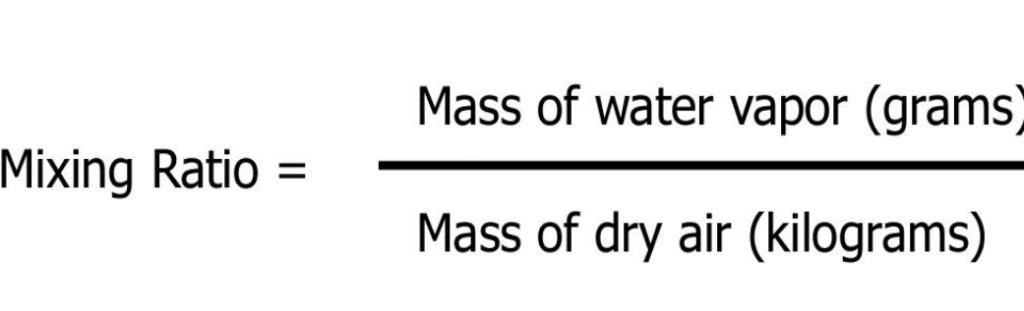
The MASS of water vapor in a unit of air compared to the remaining MASS of dry air (without water vapor)
Because mass not affected by changes in T & P, MR not affected by changes in T & P
MR = Mass fraction, (w/w)% Chemistry 102
Vapor Pressure
Total atm P attributable to its H₂O content
As more water vaporis added to dry air the vapor pressure increases
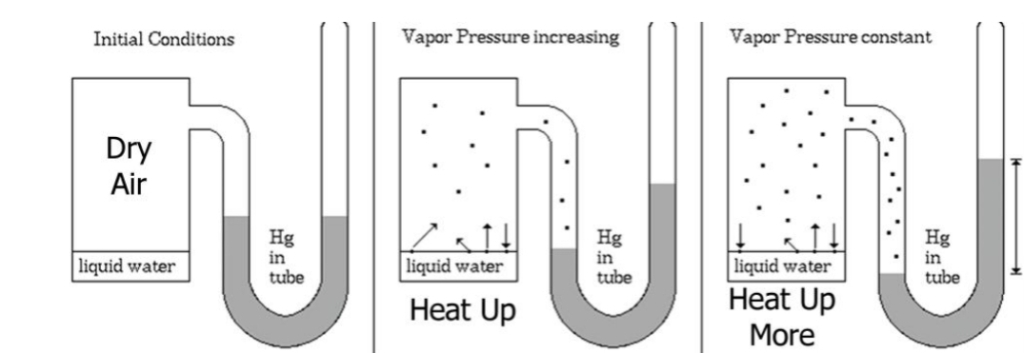
PV = nRT, n = m/Mw, R = 0.0821 Latm/molK
زيادة درجة الحرارة او الكتلة تزيد من ضغط المياه في الجو، من كيمياء 102
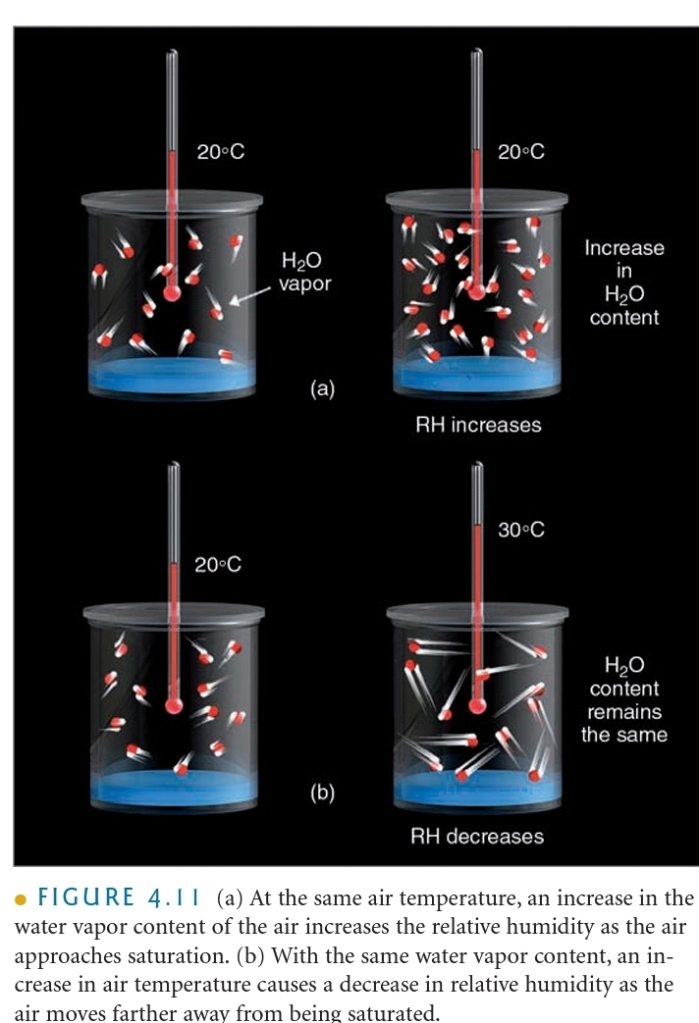
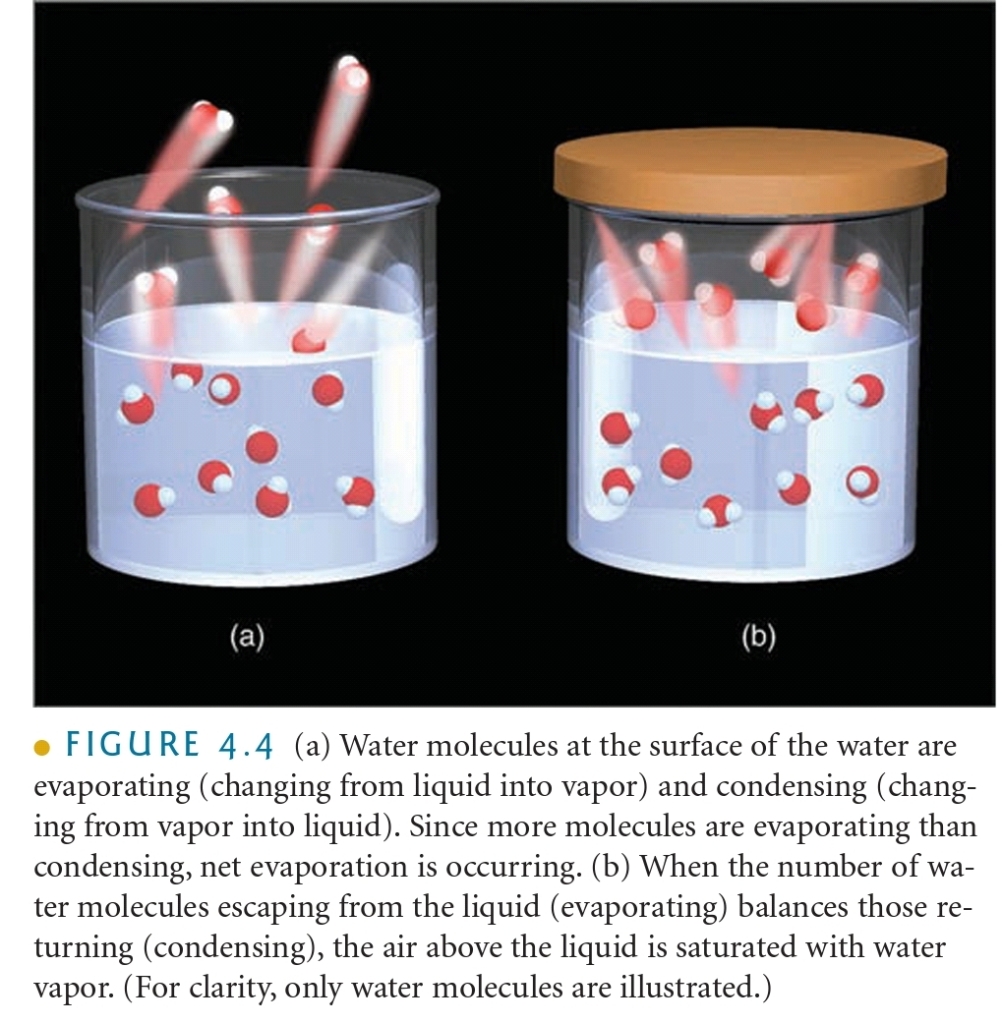
Saturation Vapor Pressure
Initially more molecules leave the surface of the water than return
SATURATION : Over time number of molecules leaving = the number molecules returning
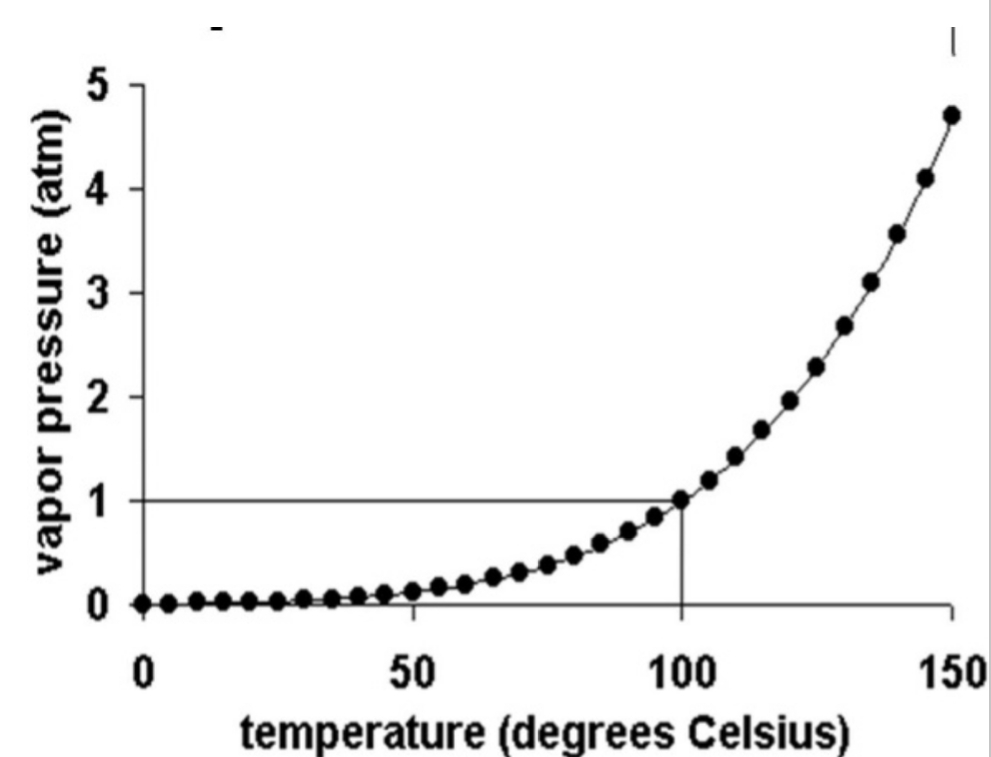
You can “FIT” more H₂O(g) in warmer air
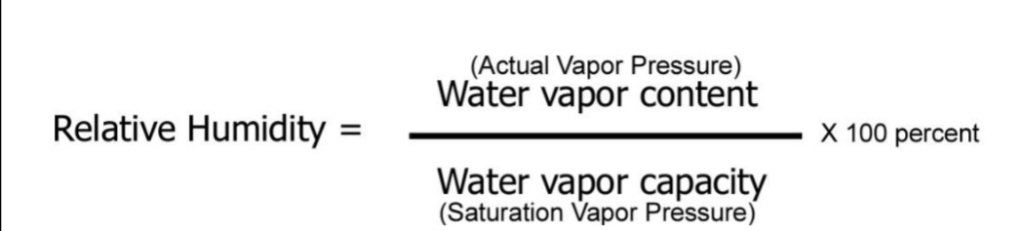
the ratio of the amount of H₂O actually in the air to the maximum amount of H₂O required for saturation at that particular T & P (ratio of the air’s H₂O content to its capacity)
RH = (SH/AH)100% = (partial P/Vapor P)100%
H2Op < Vp → Evaporation
H2Op = Vp → Saturation
H2Op > Vp → Condensation
common way of describing atm moisture
Tells us how close to Saturation the Air is… Not how much water vapor is in the air
RH change in 2 ways:
1. Change in the air H₂O content
2. Change in the air T
Air with 100%RH is saturated because it is filled to capacity with water vapor
Air with > 100%RH is supersaturated
Calculation & Concept
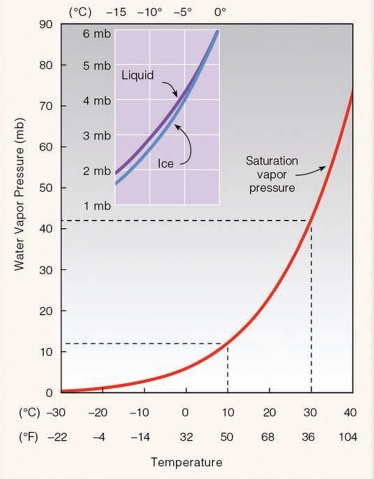
الرطوبة النسبية هي كمية الماء الحقيقية في الجو (ضغط الماء في الجو) بالنسبة للكمية القصوى لها (ضغط الماء بالجو عند الاشباع) وتتأثر في الحرارة، لنفرض اننا نريد حساب الرطوبة النسبية عند درجة حرارة 10 سيلسياس، نلاحظ من المنحنى ان عند هذه الدرجة يستوعب الهواء ضغط 12 مليون بار “الاشباع”، ولنفترض ان الجو يحتوي 10 مليون بار اذاً:
RH = (10/12)100% = 83.33% at 10°C

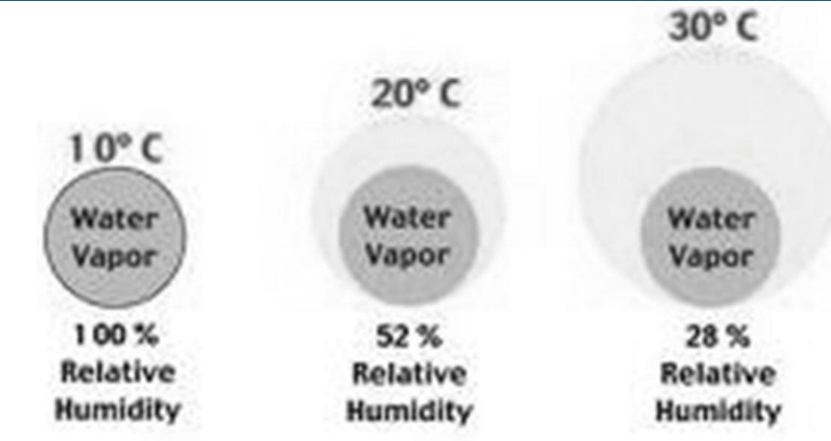

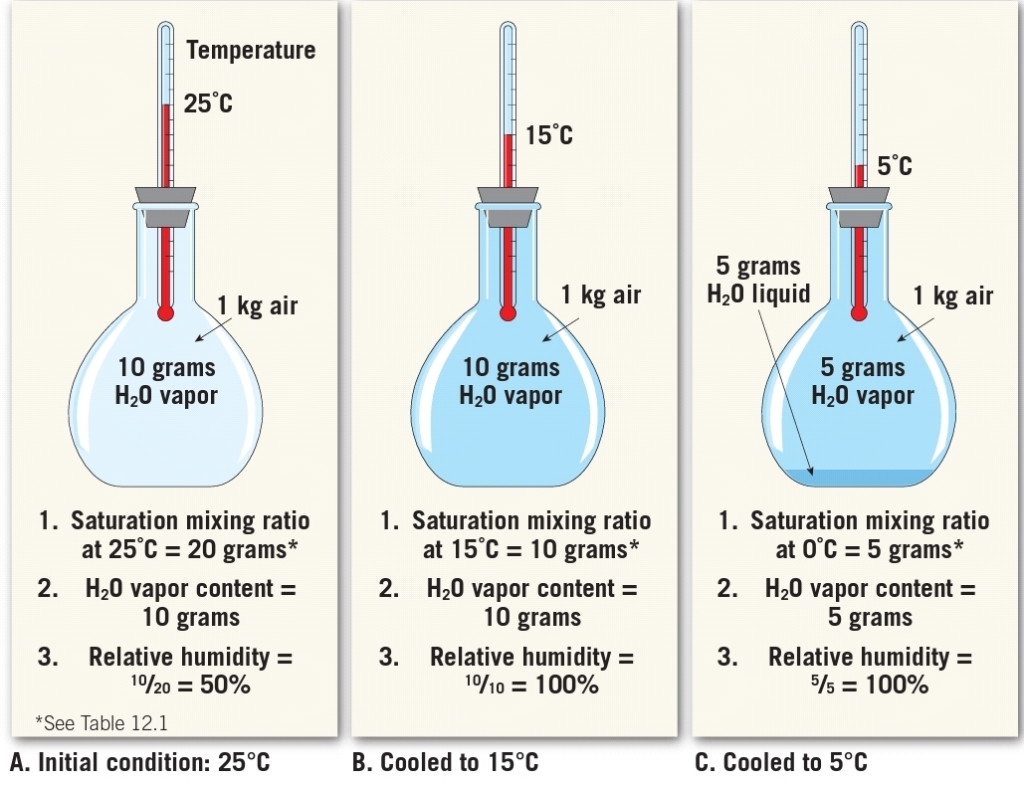
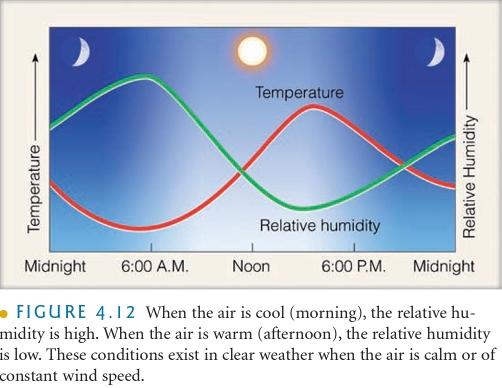
Relative Humidity, Natural Change
1. Daily changes in T (daylight verses night T)
2. T changes that result as air moves horizontally from one location to another
3. T changes as air moves vertically in atm
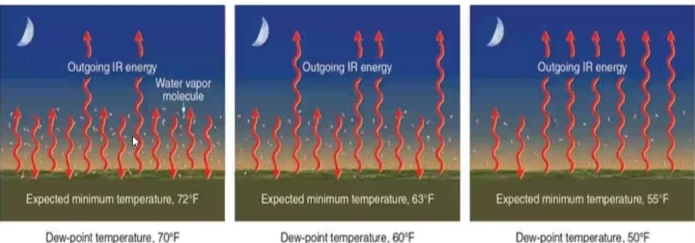
Dew Point Temperature
The temperature which air needs to be cooled to reach saturation
measure of the actual moisture content of a parcel of air
The term dew point stems from the fact that during the night objects at the surface often cool below the dew-point are coated with dew
When dew point exceeds 65Fit is considered humid by most people
dew point > 75Fit considered unbearable
Dew
The condensation of water vapor on objects that have cooled to the dew-point
– They radiated away some of their heat
– A car will get dew before the cement since they cool at different rates
– More frequent on grass since plant Transpire & release H₂O(g) right near blade!
Dew is more likely to form on nights that are clear & calm than cloudy & windy Clear nights allow objects near the ground to cool rapidly by emitting IR, & calm winds mean the coldest air will be located at ground level, These atm conditions associated with large fair-weather, & high P systems.
the cloudy, windy weather that inhibits rapid cooling near the ground & the forming of dew often signifies the approach of a rain producing storm system. & These observations inspired the following folk rhyme:
1. When the dew is on the grass, rain will never come to pass.
2. When grass is dry at morning light, look for rain before the night!
Frost
Frost is NOT frozen dew
white or hoar frost: forms from deposition

RH & HUMAN DISCOMFORT
A good measure of how cool the skin can become is the “wet-bulb T” the lowest T that can be reached by evaporating water into air
Sensible Temperature T we perceive In cold weather, when the air is calm, higher than a thermometer might indicate
Heat-related problems
Hypothalamus gland: As this perspiration evaporates, rapid loss of water & salt can result in chemical imbalance that may lead to painful heat cramps. Excessive water loss through perspiring coupled with increasing body T may result in heat exhaustion fatigue, headache, nausea, & even fainting. If body T rises above 41°C, heatstroke can occur, resulting in complete failure of the circulatory functions. If body T continues to rise, death may result
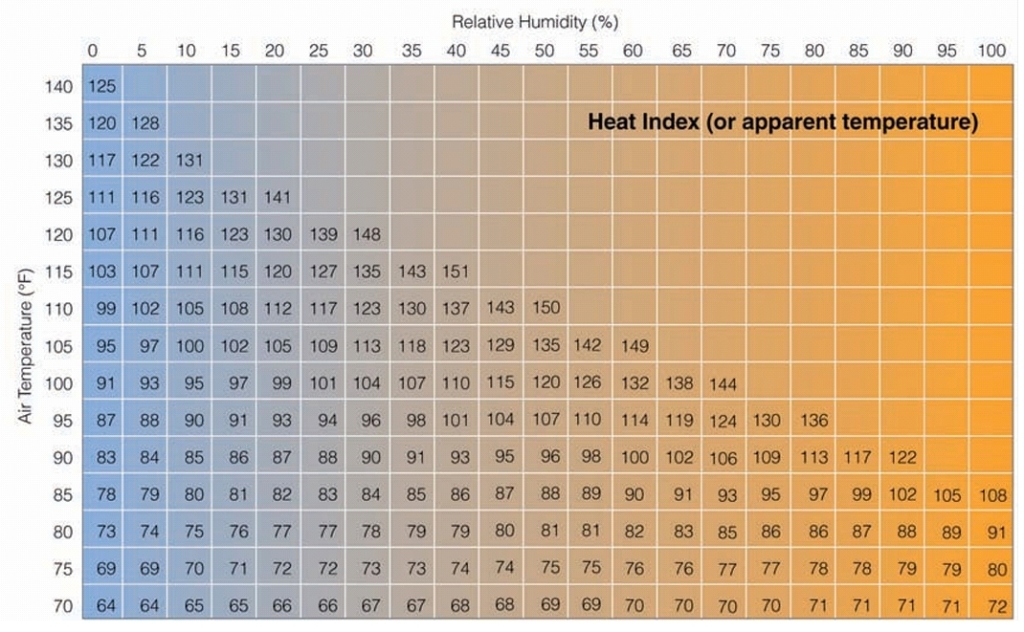
Heat Index (HI)
Index combines air T with relative humidity to determine an apparent T
what the air T “feels like” to the average person for various combination of air T & relative humidity
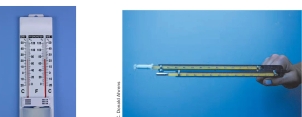
MEASURING HUMIDITY
Psychrometer Common instrument, consists of 2 liquid-in-glass thermometers mounted side by side & attached to a piece of metal that has either handle or chain at one end
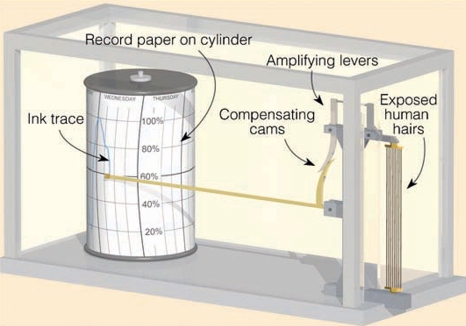
Hygrometers (Hair Hygrometer) hair to measure relative humidity, constructed on the principle “relative humidity increases, the length of hair increases”
Electrical hygrometer flat plate coated with a film of C. An electric current is sent across the plate. As H₂O absorbed, the electrical resistance of the C changes & translated into RH. commonly used in the radiosonde, which gathers atm data at various levels above Earth
Infrared Hygrometer Measures humidity by amount of IR absorbed by H₂O, & the dew cell determines the amount of H₂O in the air by measuring the air’s actual H₂O pressure
important Terms
EVAPORATION Require Energy, Process by which liquid transformed into gas, Powered by the sun
CONDENSATION Release Energy, The change from a gas to a liquid, Responsible for the formation of clouds
PRECIPITATIONF alling liquid or solid
TRANSPIRATION release of water vapor to atmosphere by plants
SUBLIMATION Require Energy, Conversion of a solid directly to a gas
DEPOSITION Release Energy, Conversion of a gas directly to a solid, without passing through an intermediate liquid phase
Humidity The general term used to describe the amount of H₂O(g) in the air
AH The MASS of water vapor in VOLUME of air
SH The MASS of water vapor in a unit of air compared to the remaining MASS of air (include water vapor) molecules
MR The MASS of water vapor in a unit of air compared to the remaining MASS of dry air
VP Total atm P attributable to its H₂O content
RH the ratio of the amount of H₂O actually in the air to the maximum amount of H₂O required for saturation at that particular T & P, or, ratio of the air’s H₂O content to its capacity
Dew Point T temperature which air needs to be cooled to reach saturation, measure of actual moisture content in air
Dew The condensation of water vapor on objects that have cooled to the dew-point
Frost NOT frozen dew, forms from deposition
Sensible T T we perceive In cold weather, when the air is calm, higher than a thermometer might indicate
wet-bulb T good measure of how cool the skin can become is the “” the lowest T that can be reached by evaporating water into air
HI Index combines air T with RH to determine an apparent T, what the air T “feels like” to the average, for various combination of air T & RH
Saturation VP When air is saturated VP exerted by motion of H₂O(g), Varies with T
Psychrometer Common instrument used to measuring RH, consists of 2 liquid in glass thermometers mounted side by side & attached to a piece of metal that has either handle or chain at one end
Hair Hygrometer measure RH, constructed on the principle “relative humidity increases, the length of hair increases”
Electrical hygrometer flat plate coated with film of C, electric current is sent across plate. As H₂O absorbed, the electrical resistance of the C changes & translated into RH
Infrared Hygrometer Measures RH by amount of IR absorbed by H₂O, & the dew cell determines the amount of H₂O in the air by measuring the air’s actual H₂O pressure
The End
