Reference: Meteorology Today 7th
Meteorology
Online Quiz
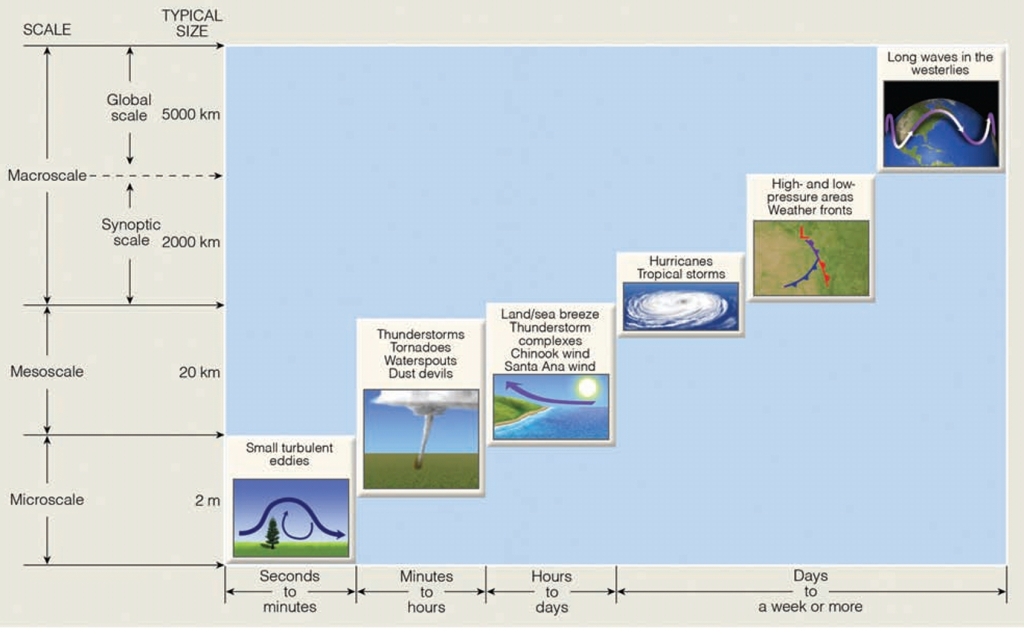
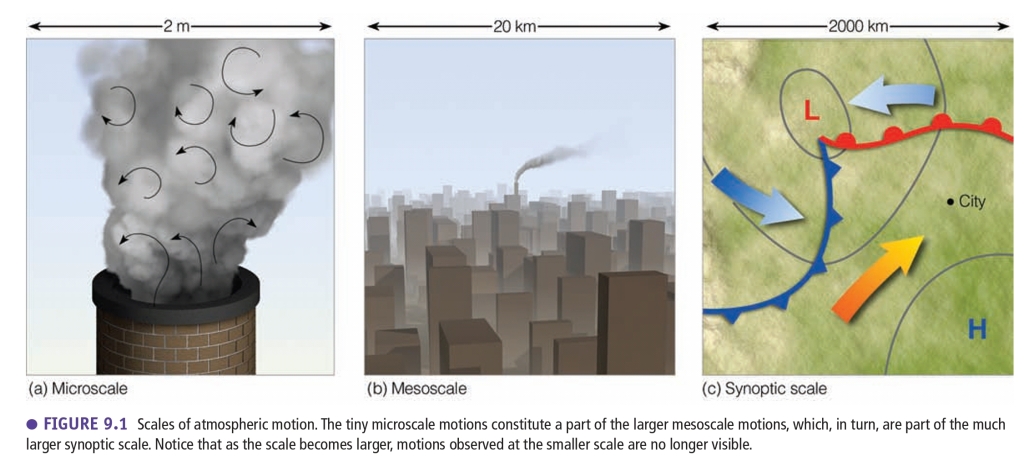
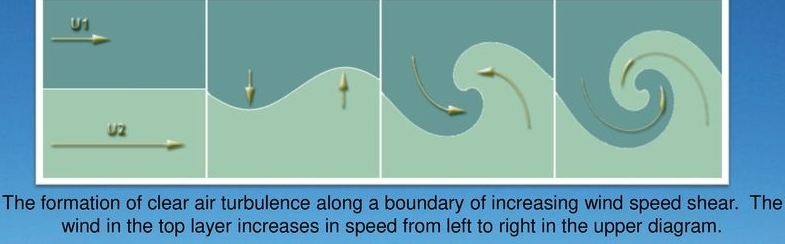
Wind : Mesoscale
Part1: Scales of Atmospheric Motion
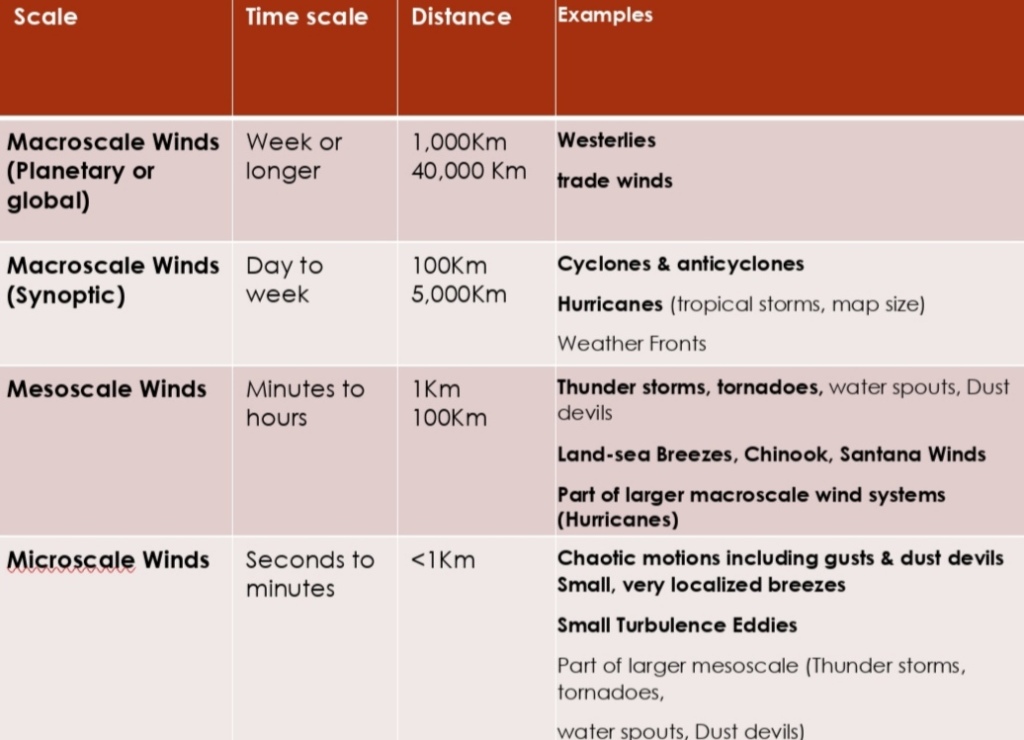
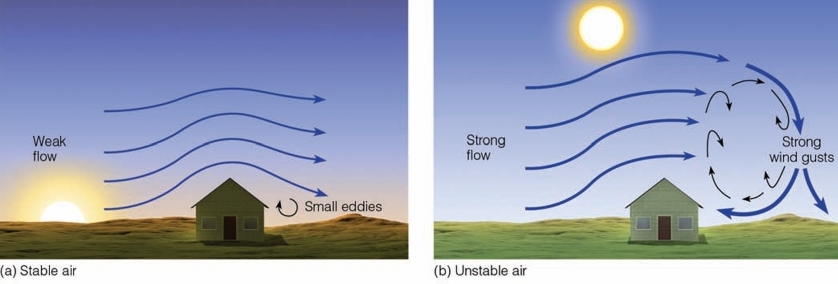
Eddy
Whirl of air, Come in different sizes
Small volume of air behaves differently from the large flow in which it resides
Eddies are down wind from the obstacle
Caused by encountering an obstacle
Part2: Mesoscale
Local Winds (Mesoscale)
Caused by topographic effects or variations in local surface composition
Include
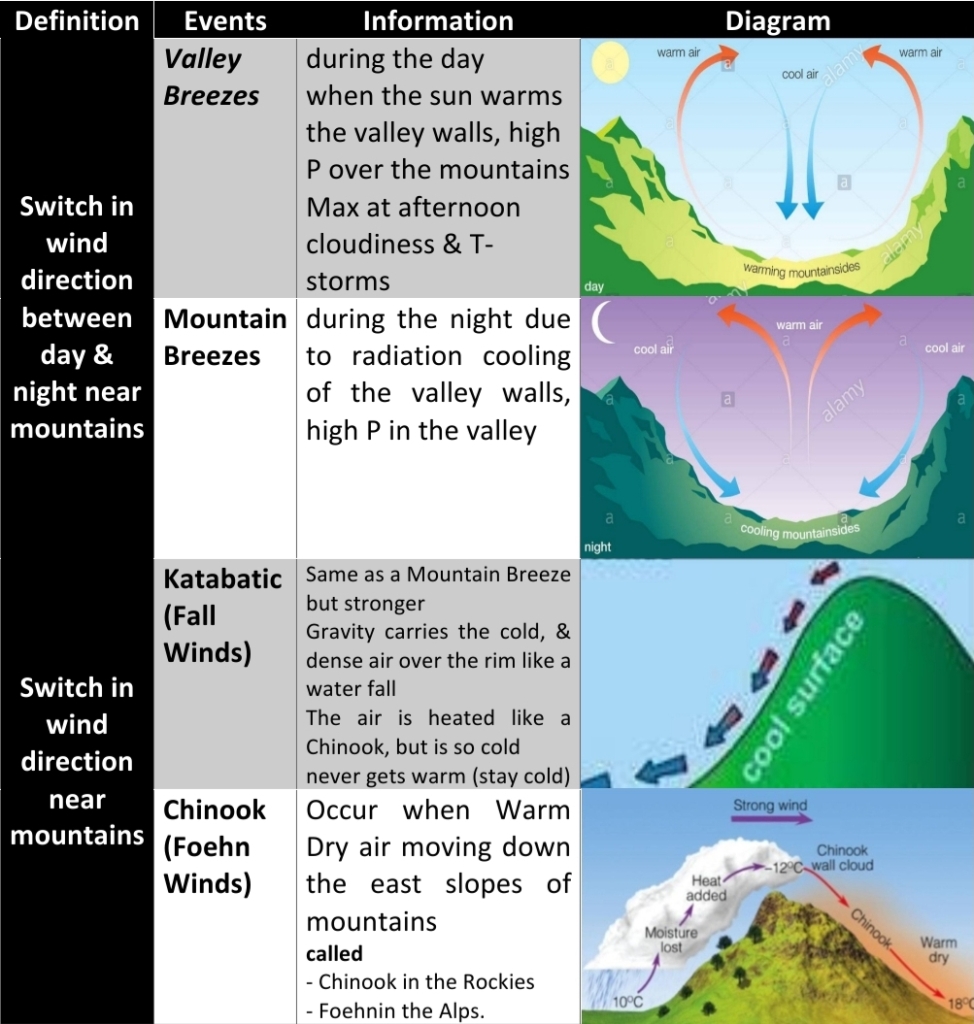
1. Land & Sea Breezes
2. Valley & Mountain Breezes
3. Chinook Winds (Foehn Winds)
4. Katabatic Winds (Fall Winds)
5. Santa Ana Winds
6. Haboobs Winds (Desert Winds)
7. Dust Devils
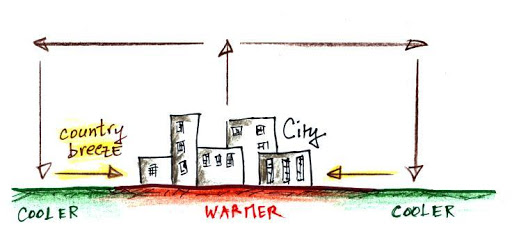
8. Country Breezes



Part3 : MONSOON
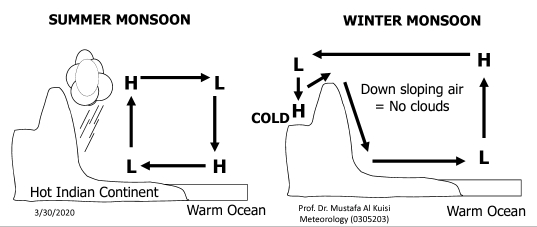
MONSOON
Seasonal change in global circulation
Refers to Seasonal reversal of wind direction
Caused by Alternation between 2 weather patters
Doesn’t mean rainy season

The End
Online Quiz
Stability & Cloud Development
Adiabatic Lapse Rates
Adiabatic process
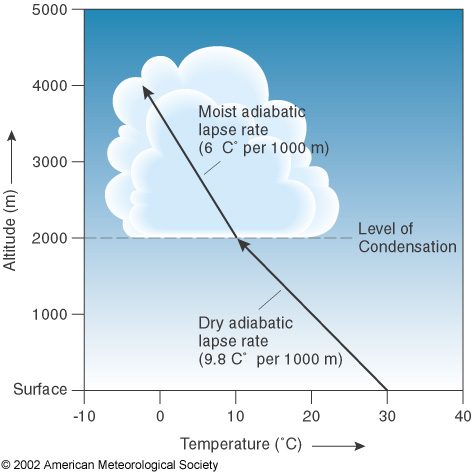
dry adiabatic rate DAR = 10°C/Km
If parcel of unsaturated air expands & cools, or compresses & warms, with no interchange of heat withits surroundings
The Rate of adiabatic cooling or warming remains constant “only to unsaturated air”
Wet adiabatic rate WAR ≈ 6°C/Km
As the rising air cools, its RH increases, & at the Td, RH becomes 100%
Further lifting results in condensation, a cloud forms, & latent heat is released inside the rising air parcel
The air no longer cools at the DAR but at a lesser rate. Because the heat added offsets some of the cooling due to expanding
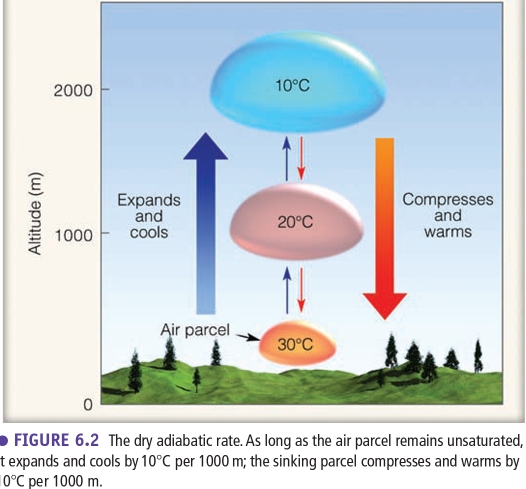
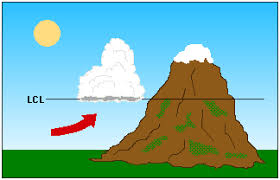
Lifted Condensation Level (LCL)
The height at which air that is cooling at the DAR becomes saturated & condensation begins
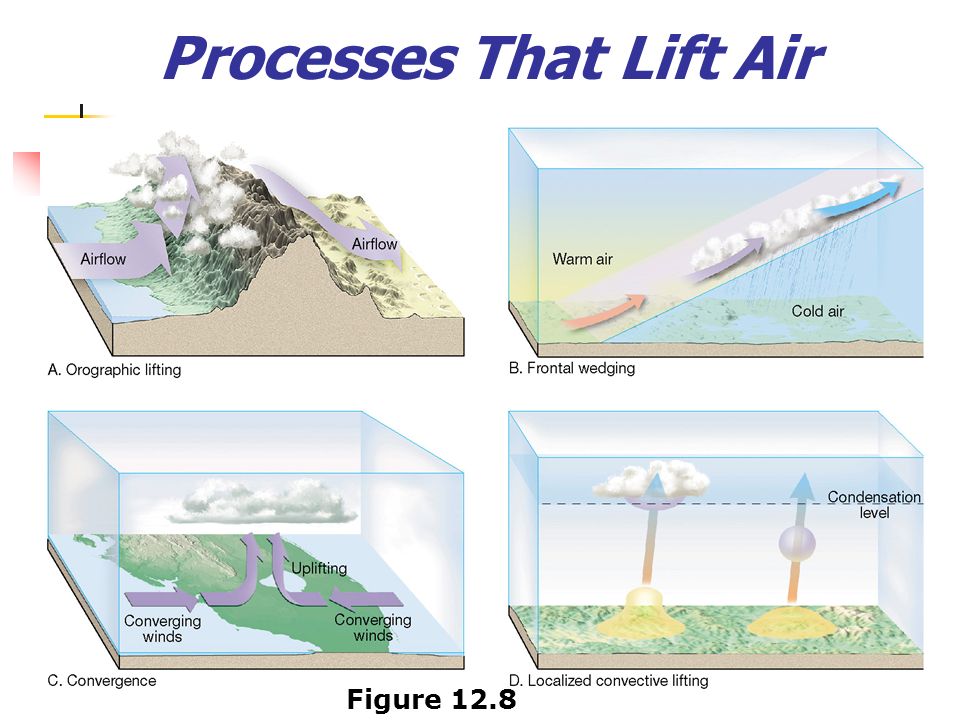
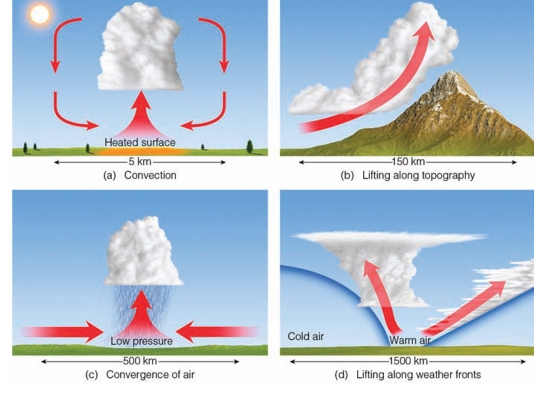
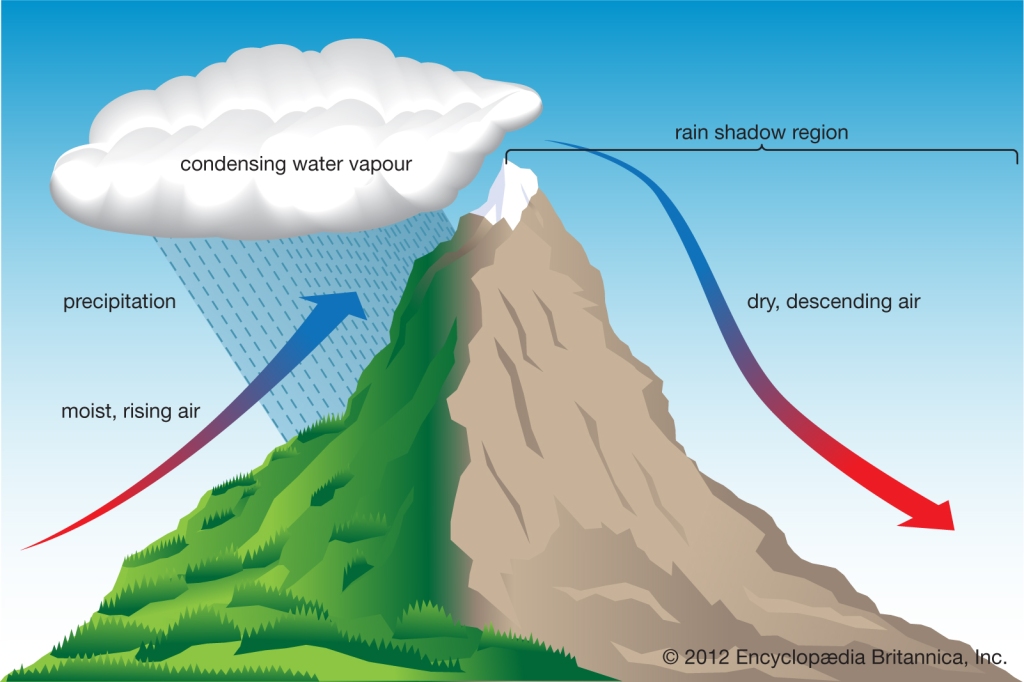
Processes that Lift Air
Orographic Lifting: forced over a mountain
Convergence forced to rise as it collides
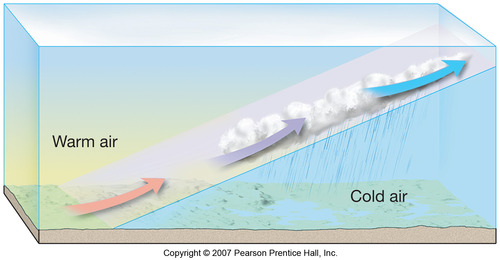
Frontal Wedging: air is forced up due to difference in air T or ρ
Localized Convective Lifting differential heating, Air is forced to rise to heating air & lowering its density
Air Parcels
Imaginary volume of air
– a few hundred m³
ASSUMPTION
1. no heat is transferred into, or out of it
2. Acts independently of the surrounding air
3. HIGHLY IDEALIZED
We use them to
1. Determines if the air will rise or sink by comparing parcel of air to its surrounding
2. predict if clouds will form
Adiabatic Lapse Rates ALR
WALR & DALR help us to
1. Understand if a parcel will rise or sink
1. Determines if a cloud will form
2. Determines type, & height of clouds


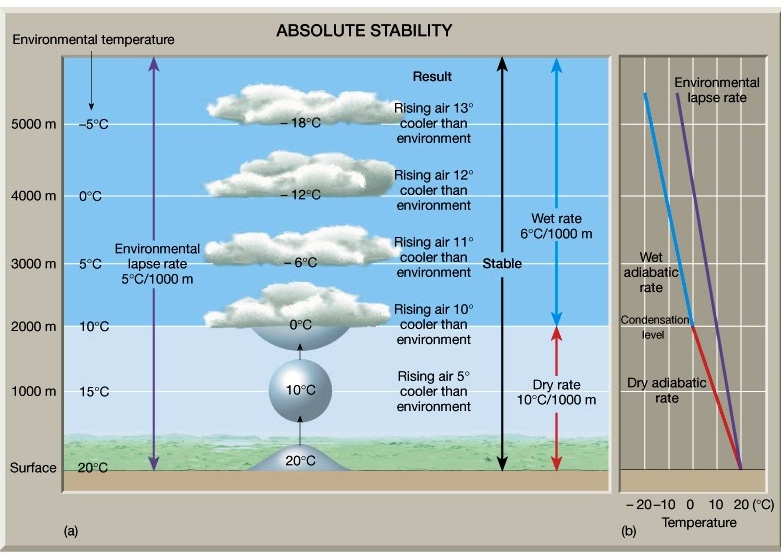
Environmental Lapse Rate (ELR)
Actual measureable T change with height
Atmospheric stability
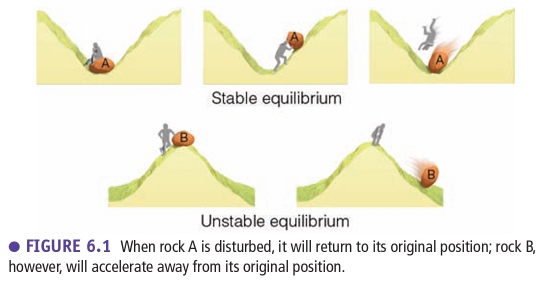
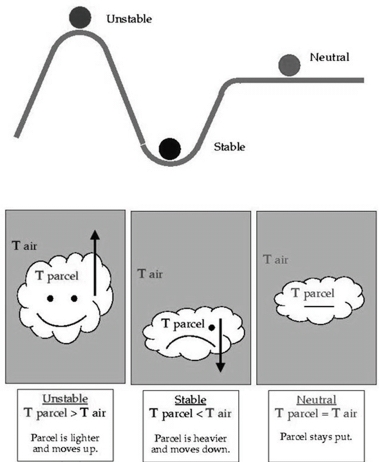
Stable Air (Tair < Tsur.)
If a parcel cooler than the surrounding, it would be more dense, so sink back to it’s original position
Air resists vertical (up & down) motion
Air is in stable equilibrium when it tends to return to its original position
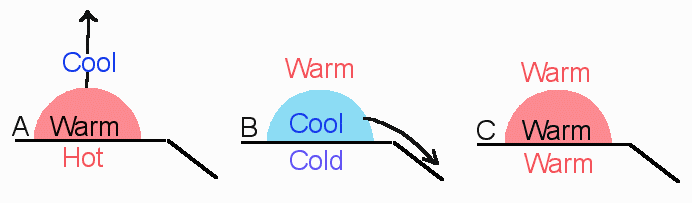
Unstable Air (Tair > Tsur.)
If a parcel warmer than the surrounding, it would be less dense, so rise to an altitude where it’s T equaled that of surrounding
Air that is in unstable equilibrium will move away from its original position

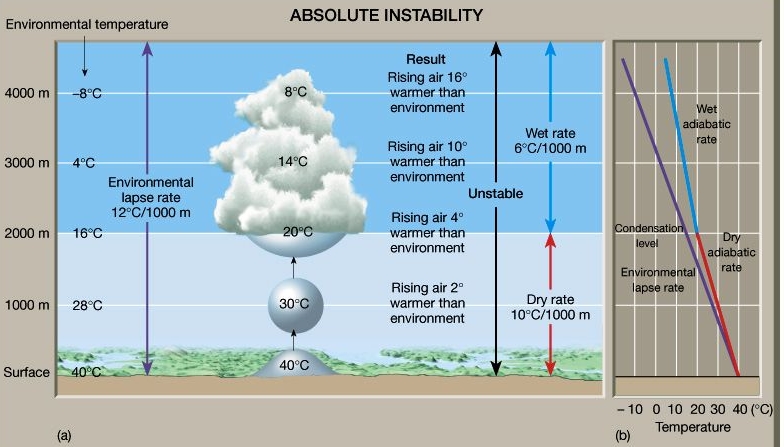

Stability & Daily Weather
If stable air is forced up, the associated clouds have little vertical thickness & precipitation is light
Clouds associated with unstable air are towering & accompanied by heavy rain
Thunderstorms produced by the unstable air that caused by the passing hurricanes
Changes in Stability
Stability is enhanced by the following
1. Radiation cooling of surface after sunset
2. The cooling of an air mass from below
3. General subsidence within an air column (sinking)
Stable atmosphere are cooling from below & warming aloft
Instability is enhanced by the following
1. Solar heating warming the lower atm
2. The heating of an air mass from below
3. Upward movement of air
4. Radiation cooling from cloud tops
Unstable atmosphere are heating from below & colling aloft
Vertical Air Movement
Subsidence downward motion of air
Stabilizes the air if the air above is warmed
Can result in the evaporation of clouds
Summary
ALR
DALR = 10°C/Km, Non-Condensing air
WALR ≈ 6°C/Km, Condensing air
Atm Stability
Stable air : Tp < Ta, sink
Unstable air : Tp > Ta, rises
Neutral : Tp = Ta, stay in its hight
Types of Stability
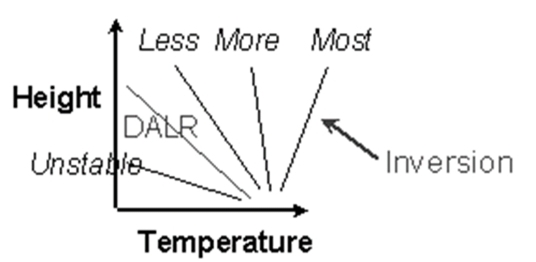
Absolute Stability:
ELR < WALR, ELR < 6°C/Km
Absolute Instability:
ELR > DALR, ELR > 10°C/Km
Conditional Instability :
DALR > ELR > WALR, 10 >ELR> 6
The End
Online Quizzes
1st : Haze & Foge
2nd : Clouds
Condensation
Haze & Fog
Haze
layer of dust or salt particles suspended above the region
tiny dry haze particles scatter some rays of sunlight, & allowing others to penetrate air.
The scattering effect of dry haze
1. bluish color when viewed against a dark- colored background
2. yellowish when viewed against a light- colored background
As the air cools during the night, the RH increases, & When the RH = 75٪, condensation may begin on the most active hygroscopic nuclei, producing a wet haze
FOG
cloud with its base at or near the ground
Types of Fog:
Fogs formed by Cooling
1. Radiation
2. Advection
3. Upslope
Fogs formed by Evaporation
1. Steam
2. Frontal
Radiation Fog
Fog formed During the night, when The ground cools the air close to the surface
– Tend to form on clear, relative calm night when cool, moist air is overlain by drier air & rapid Radiational Cooling occurs
– Clear Skies
– Fairly high RH
– Cold air sinks in valleys
– Dissipate يبدد after sunrise

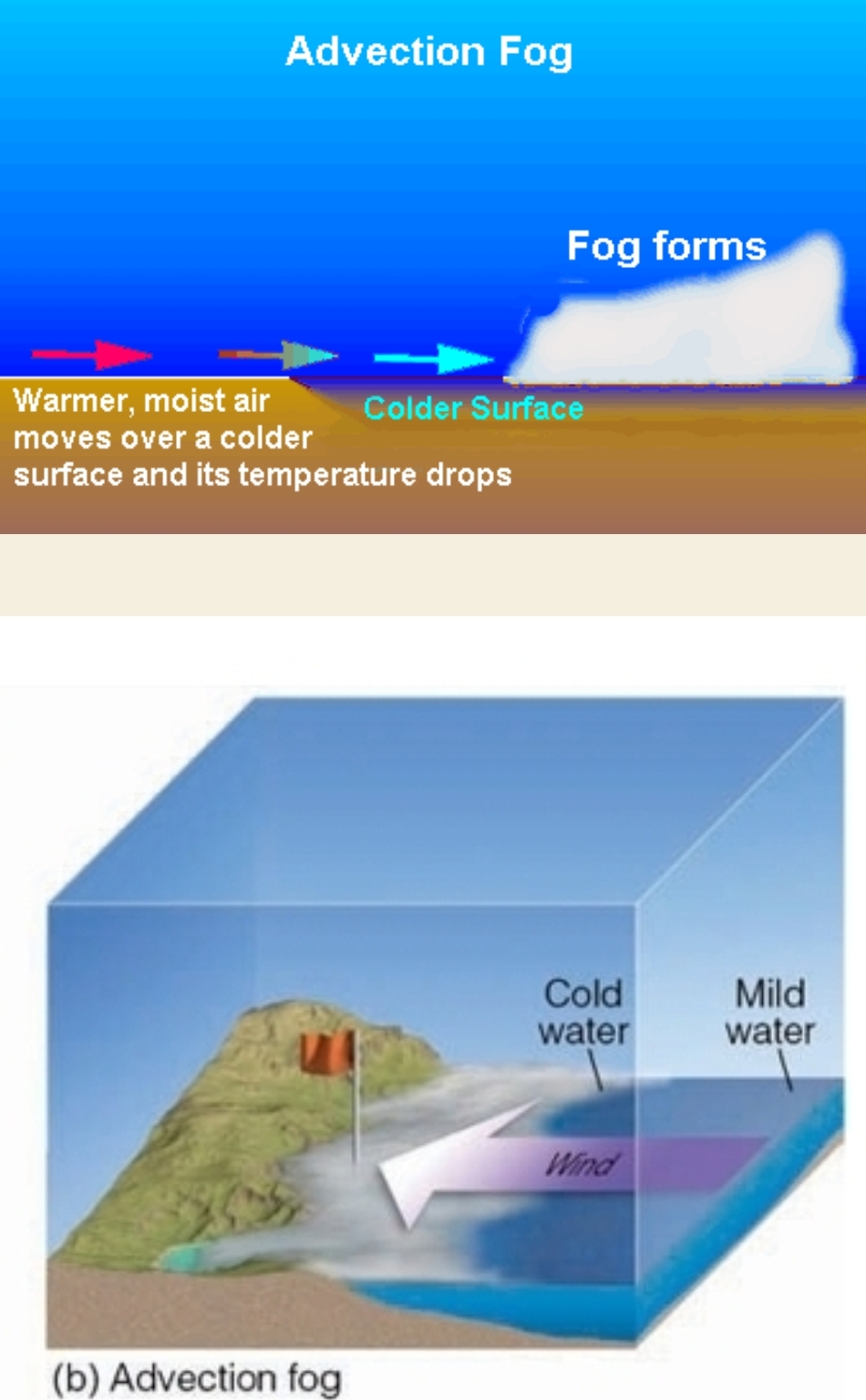
Advection Fog
Fog formed by horizontal movement of air, if Warm moist air is blown over a cold surface
– Advection = horizontal movement
– Turbulence (wind) is needed to make it thick
Upslope Fog
Fog formed as humid air moves up a gradual sloping plain or mountain (or as Moist air rises, cools, & condenses over elevated terrain)
– it cools as moves up, formed at dew point
– Most likely to form along an Irregular Coastline at the headland
– headland: region of land extended seaward
– Ex: Great Plains

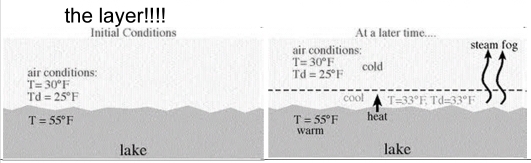
Steam Fog
Fog formed as Cool air moves over warm water
– Rising air cools & condenses
– Moister added into the layer

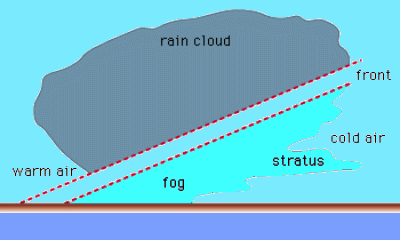
Frontal Fog
as warm air is pushed over a colder one
– If cold air is near dew, point rain evaporate & produce fog
– Results addition of H₂O(g) into cool air layer
Fog That Forms by Mixing
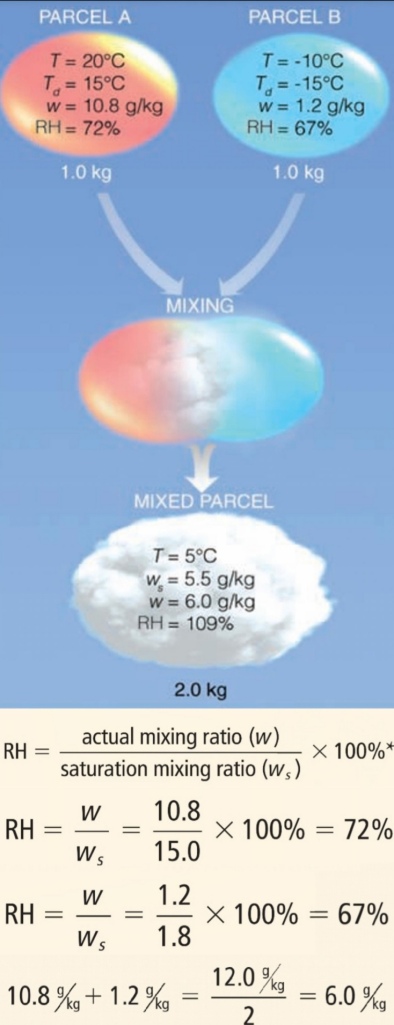
clouds
cloud
any visible aggregate of tiny droplets of water or ice crystals, or mixture of both
Cloud drops, on average 10 um in diameter
Come in a variety of shapes & sizes
Found at a large range of altitudes from the surface to the stratosphere
Help meteorologists figure out what’s going on in the atmosphere
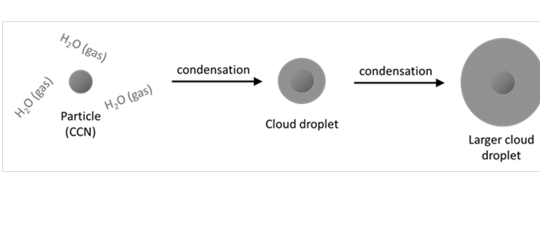
Recipe for a Cloud
1. Cloud Condensation Nuclei (CCN): the surface for water vapor to condense upon
2. Rising Air: Causes adiabatic cooling
3. Water Vapor

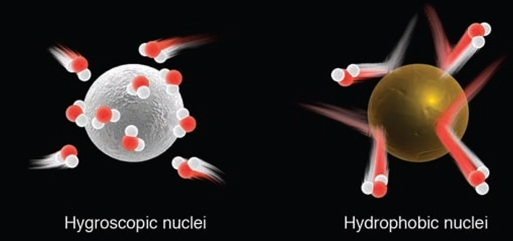
Why do you need CCN?
Without CCN the RH would have to be much greater than 100% to form a cloud drop
hygroscopic particles is water-seeking or liking, vapor rapidly condenses on their surfaces, such as Sea salt
Hydrophobic Particles water repelling, resist condensation, can still serve as nuclei when RH is > 100%
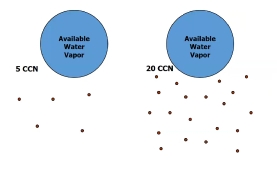
Why are cloud drops so TINY?!
The drops grow FAST & other aerosol want water too
H₂O is split up over a lot of little dropsinstead of a few big ones

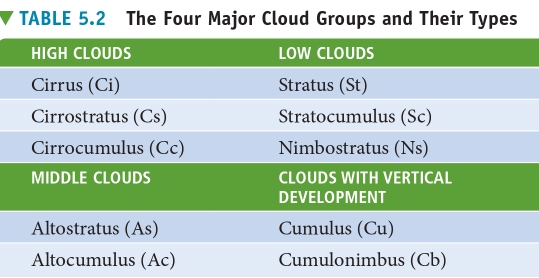
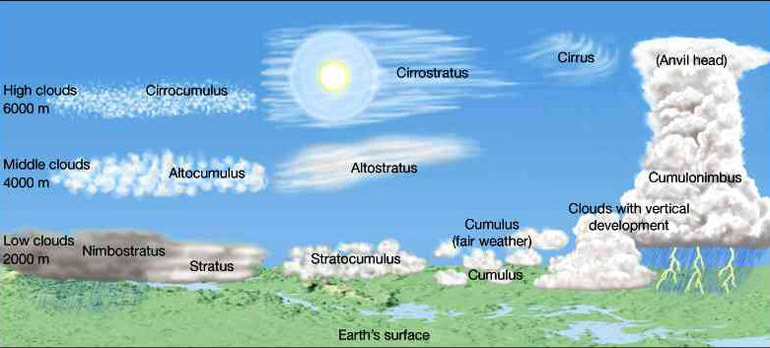
Cloud Classification
History & Classification System
Ancient astronomers named the major stellar constellations about 2000 years ago
Lamarck proposed the first system for classifying clouds in 1802
One year later, L.Howard, developed a cloud classification system that found general acceptance
Howard’s system used Latin ‘“words” to describe clouds as they appear to a ground observer
1. sheet-like cloud = stratus = layer
2. puffy cloud = cumulus = heap
3. wispy cloud = cirrus = curl of hair
4. rain cloud = nimbus = violent rain
These system has 4 basic cloud forms, & Other clouds described by combining basic types.
nimbostratus : rain cloud, shows layering
cumulonimbus : rain cloud having pronounced vertical development (puffy)
Then, Abercromby & brandsson published a classification system that is still in use today
Abercromby-brandsson system
include:
– 10 principal cloud forms
– 4 primary cloud groups
Each group identified by:
1. the height of the cloud base
2. their appearance
Groups
1. high clouds
2. middle clouds
3. low clouds
4. vertical clouds

The End
Humidity, Condensation, Clouds
Online Quiz

Special Properties of water
– easily changes from solid to liquid to gas
– Ice is LESS dense than water so it floats (Ice cubes, ice bergs, & ice caps)
– Has an unusually high heat capacity (3 times that of land)
– The angle in the water molecule = 104°
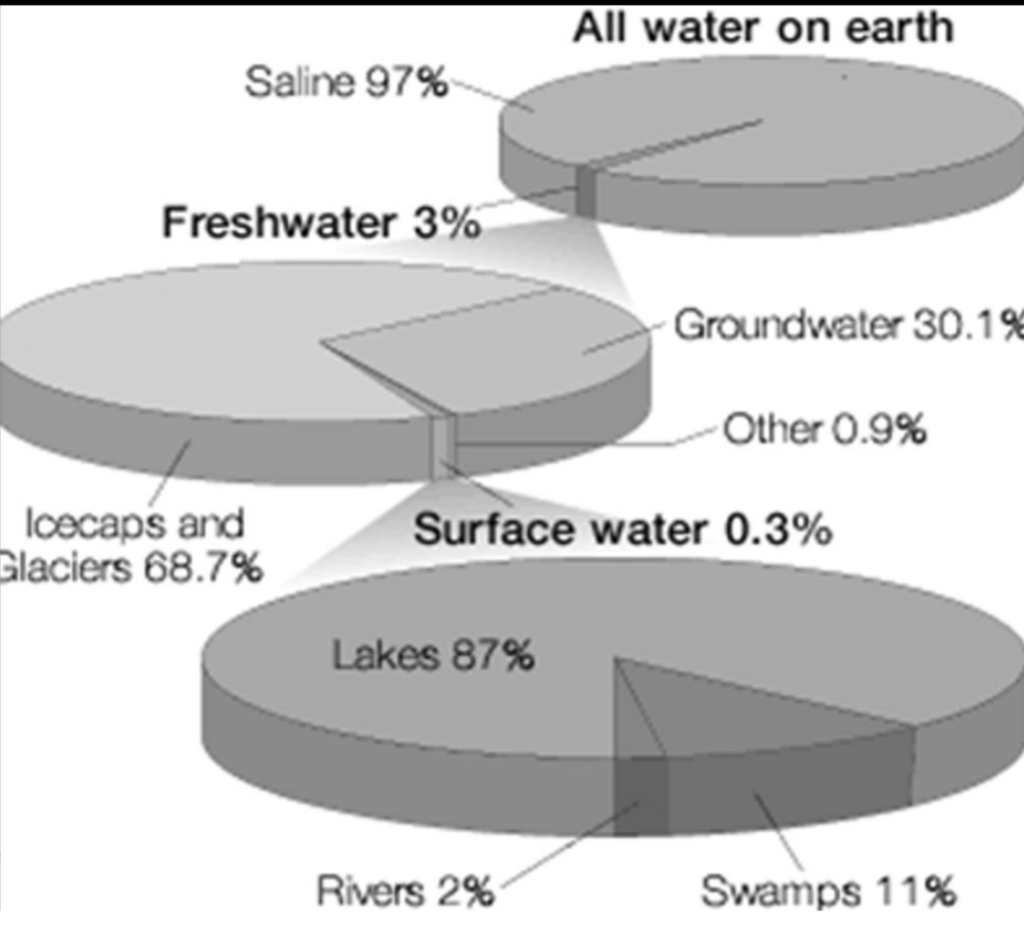
body of the earth is made up of 70% H₂O
Oceans account for most of water (>97%)
Ice sheets in Antarctica & Greenland (< 3%)
Atmosphere has only a little (0.001%)
EVAPORATION (Require Energy)
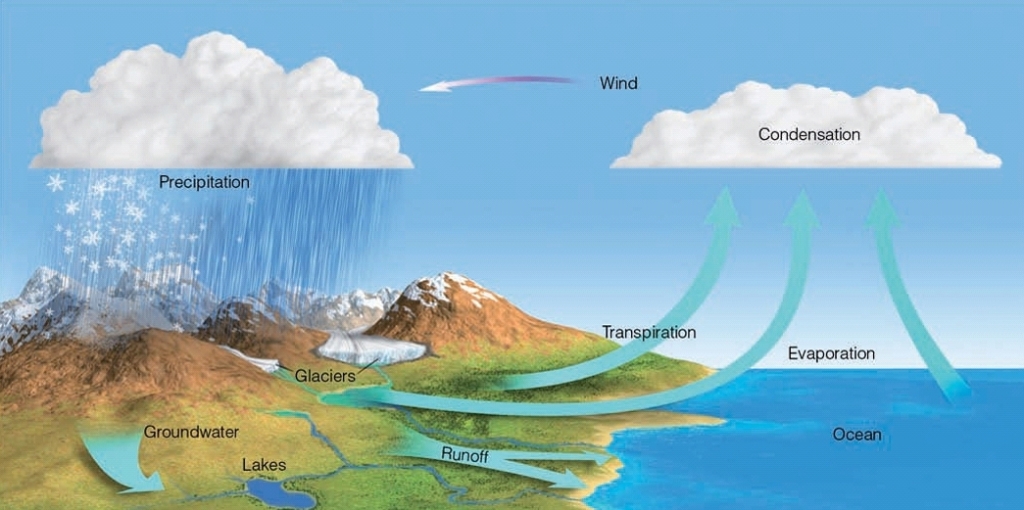
Process by which liquid transformed into gas
Happens over Oceans, Lakes, Rivers & other “standing” bodies of water
Powered by the sun: Solar radiation heats up water molecules until they are “freed” from the liquid state
CONDENSATION (Release Energy)
The change from a gas to a liquid
Responsible for the formation of clouds
PRECIPITATION
Falling liquid or solid in the atmosphere
Balances Evaporation: Average annual precipitation equals evaporation
Happens over Land or Oceans
Returns the water to the ocean or soaks into the ground
TRANSPIRATION
release of H₂O(g) to atmosphere by plants
Plants uptake water through their roots that fell as precipitation
Not as important as evaporation
SUBLIMATION (Require Energy)
Conversion of a solid directly to a gas
EX: Gradual shrinking of unused ice cubes, the rapid conversion of dry ice into gas.
How piles of snow tend to disappear even if air T never reaches above 32F (if air is dry)
DEPOSITION (Release Energy)
Conversion of a gas directly to a solid, without passing through an intermediate liquid phase
EX: Frost on a window pane (white frost, hoar frost… FROST)
EX: Frost the builds up in the freezer.. Was once part of your ice cube!
Location of Processes
Evaporation exceeds Precipitation over Water (No plants), so no transpiration
Precipitation exceeds Evaporation over Land
Condensation happens everywhere
Humidity
The general term used to describe the amount of H₂O(g) in the air

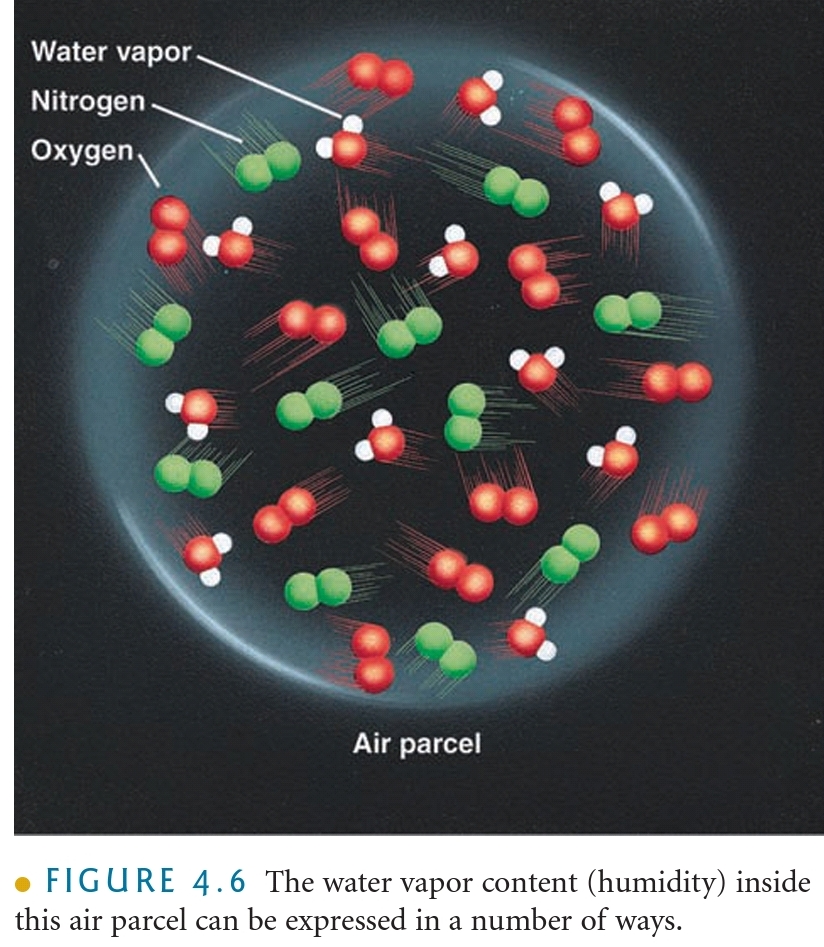
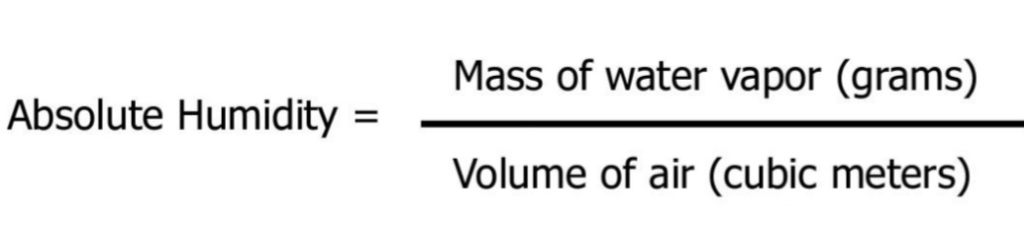

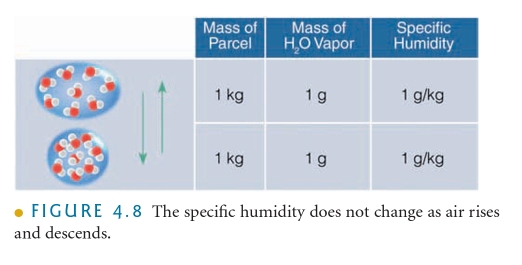
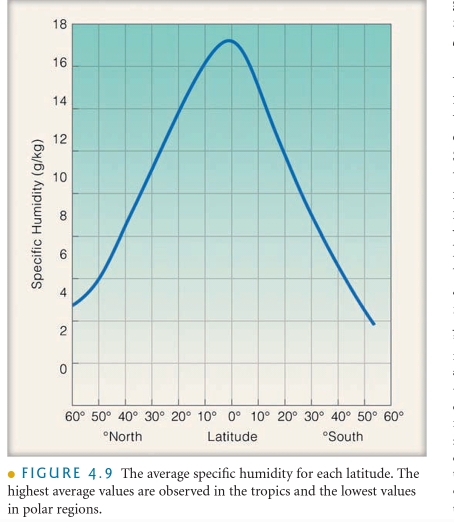
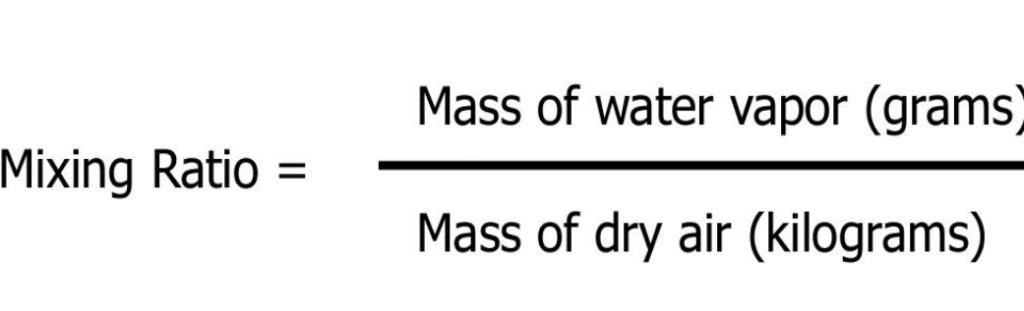
Vapor Pressure
Total atm P attributable to its H₂O content
As more water vaporis added to dry air the vapor pressure increases
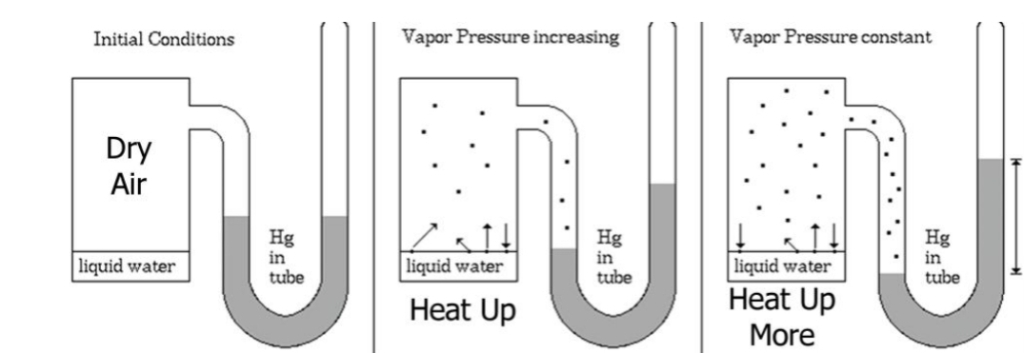
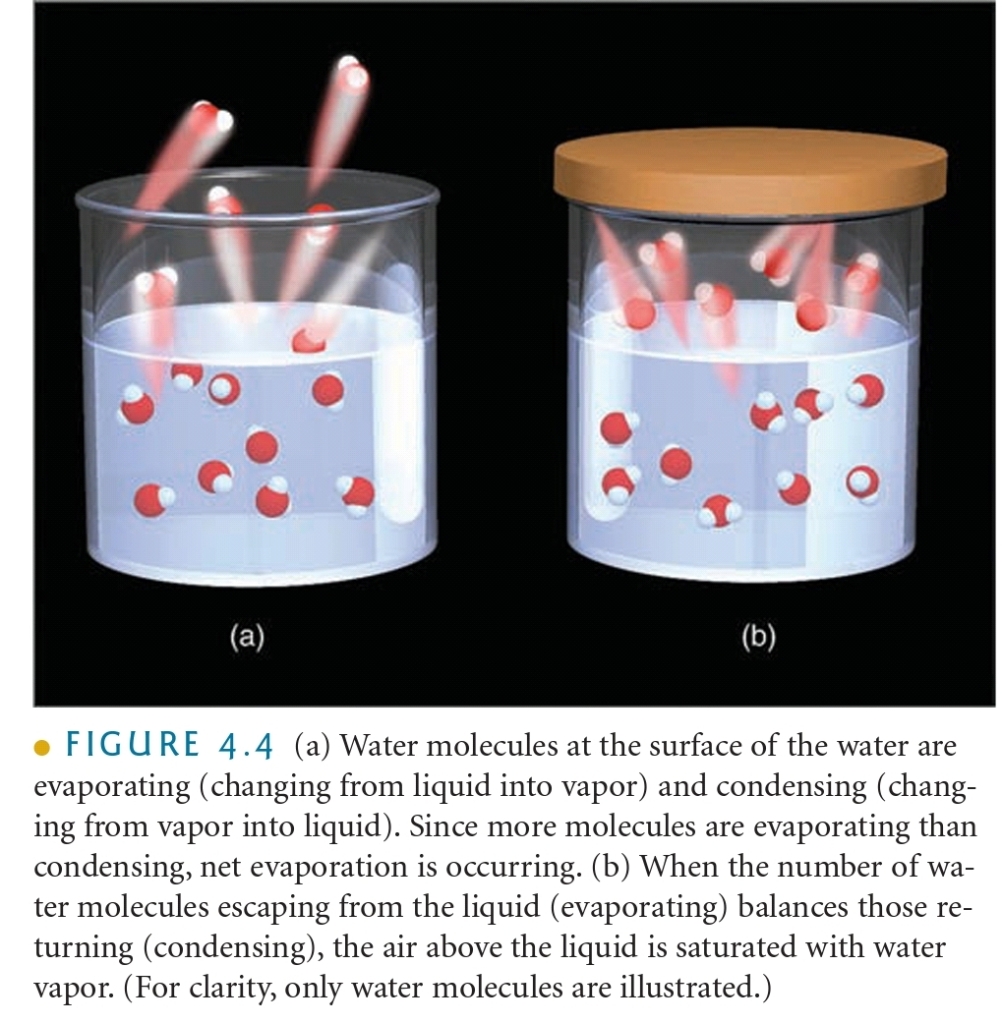
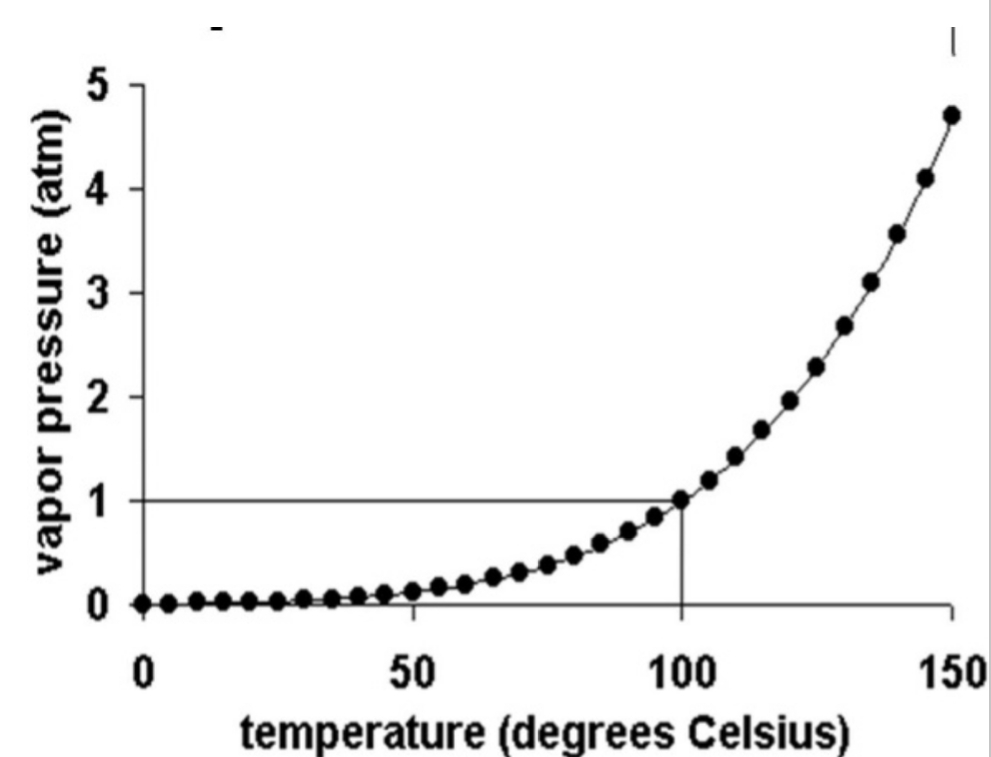
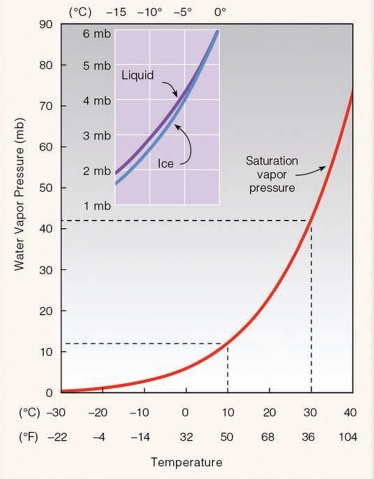
Saturation Vapor Pressure
Initially more molecules leave the surface of the water than return
SATURATION : Over time number of molecules leaving = the number molecules returning

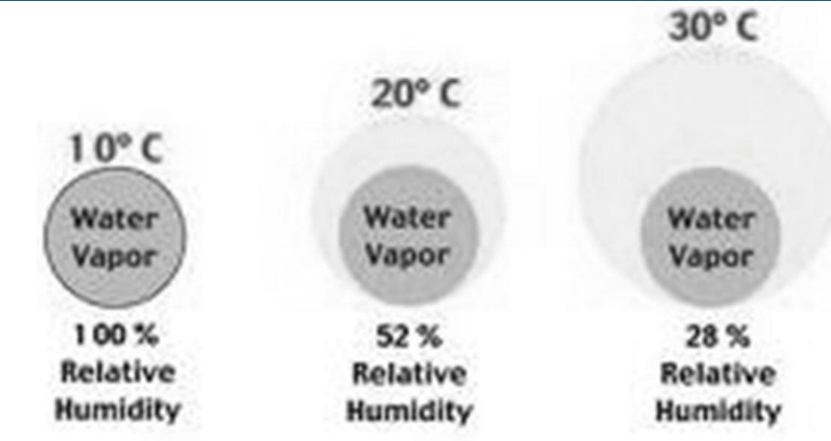

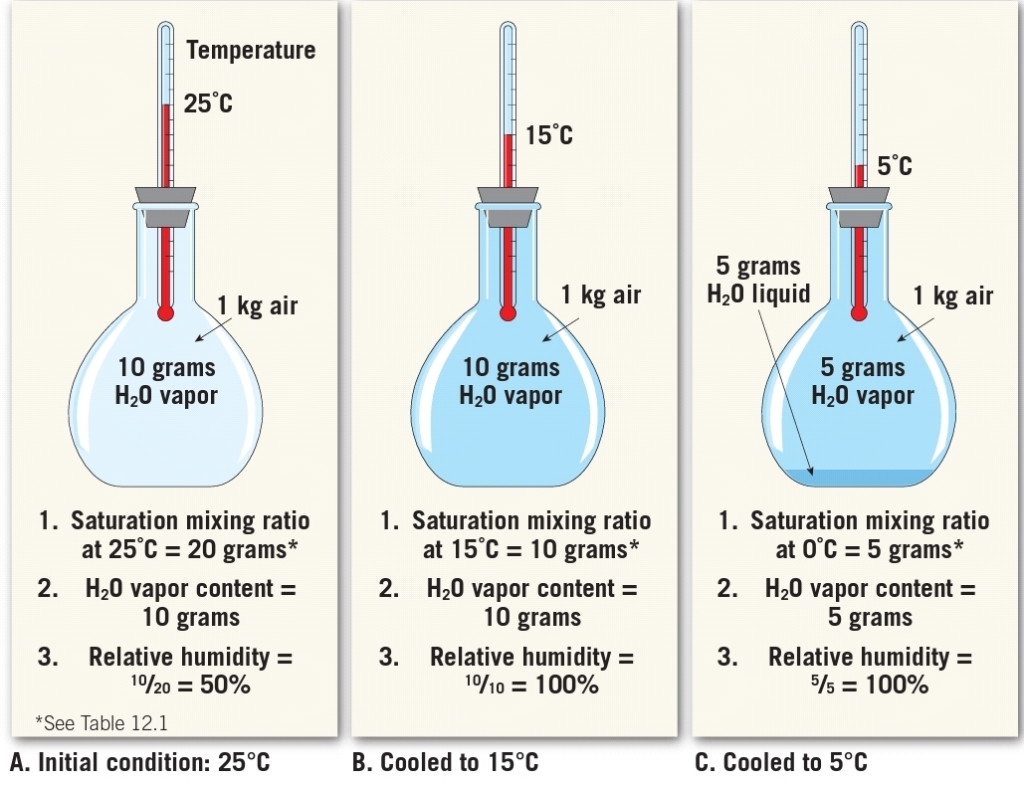
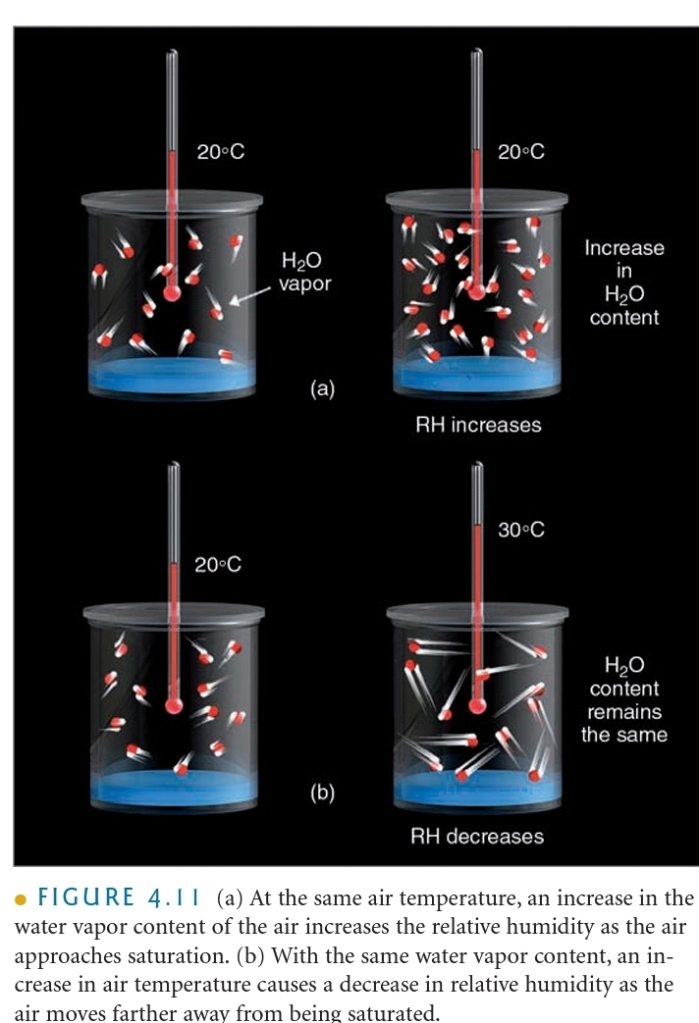
Relative Humidity, Natural Change
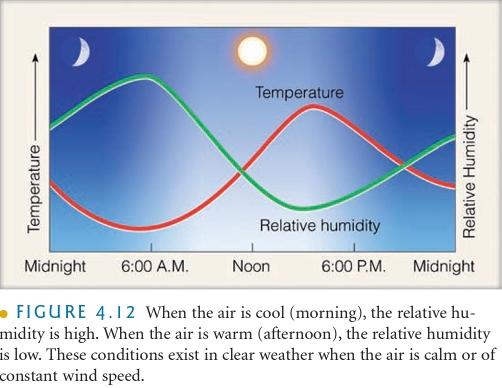
1. Daily changes in T (daylight verses night T)
2. T changes that result as air moves horizontally from one location to another
3. T changes as air moves vertically in atm
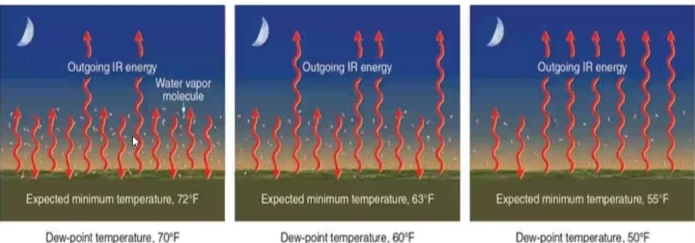
Dew Point Temperature
The temperature which air needs to be cooled to reach saturation
measure of the actual moisture content of a parcel of air
The term dew point stems from the fact that during the night objects at the surface often cool below the dew-point are coated with dew
When dew point exceeds 65Fit is considered humid by most people
dew point > 75Fit considered unbearable
Dew
The condensation of water vapor on objects that have cooled to the dew-point
– They radiated away some of their heat
– A car will get dew before the cement since they cool at different rates
– More frequent on grass since plant Transpire & release H₂O(g) right near blade!
Dew is more likely to form on nights that are clear & calm than cloudy & windy Clear nights allow objects near the ground to cool rapidly by emitting IR, & calm winds mean the coldest air will be located at ground level, These atm conditions associated with large fair-weather, & high P systems.
the cloudy, windy weather that inhibits rapid cooling near the ground & the forming of dew often signifies the approach of a rain producing storm system. & These observations inspired the following folk rhyme:
1. When the dew is on the grass, rain will never come to pass.
2. When grass is dry at morning light, look for rain before the night!
Frost
Frost is NOT frozen dew
white or hoar frost: forms from deposition

RH & HUMAN DISCOMFORT
A good measure of how cool the skin can become is the “wet-bulb T” the lowest T that can be reached by evaporating water into air
Sensible Temperature T we perceive In cold weather, when the air is calm, higher than a thermometer might indicate
Heat-related problems
Hypothalamus gland: As this perspiration evaporates, rapid loss of water & salt can result in chemical imbalance that may lead to painful heat cramps. Excessive water loss through perspiring coupled with increasing body T may result in heat exhaustion fatigue, headache, nausea, & even fainting. If body T rises above 41°C, heatstroke can occur, resulting in complete failure of the circulatory functions. If body T continues to rise, death may result
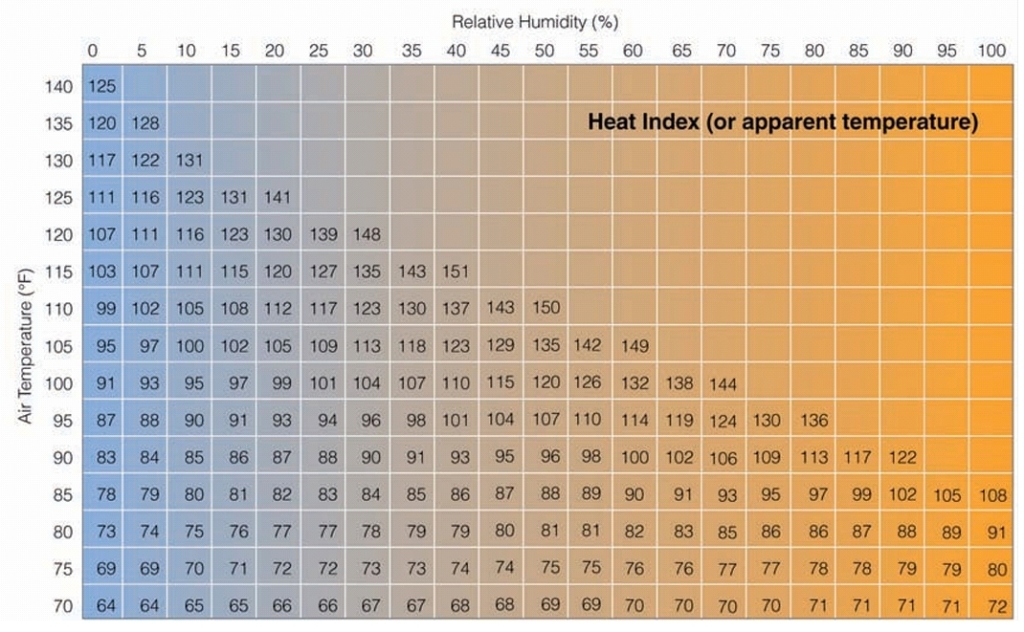
Heat Index (HI)
Index combines air T with relative humidity to determine an apparent T
what the air T “feels like” to the average person for various combination of air T & relative humidity
MEASURING HUMIDITY
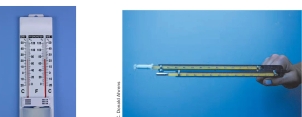
Psychrometer Common instrument, consists of 2 liquid-in-glass thermometers mounted side by side & attached to a piece of metal that has either handle or chain at one end
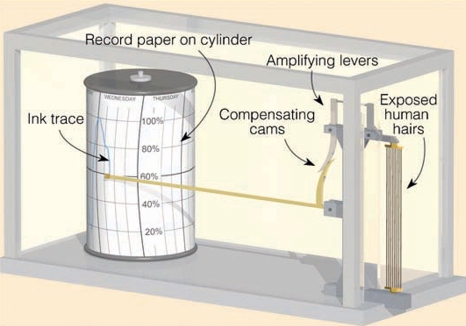
Hygrometers (Hair Hygrometer) hair to measure relative humidity, constructed on the principle “relative humidity increases, the length of hair increases”
Electrical hygrometer flat plate coated with a film of C. An electric current is sent across the plate. As H₂O absorbed, the electrical resistance of the C changes & translated into RH. commonly used in the radiosonde, which gathers atm data at various levels above Earth
Infrared Hygrometer Measures humidity by amount of IR absorbed by H₂O, & the dew cell determines the amount of H₂O in the air by measuring the air’s actual H₂O pressure
important Terms
EVAPORATION Require Energy, Process by which liquid transformed into gas, Powered by the sun
CONDENSATION Release Energy, The change from a gas to a liquid, Responsible for the formation of clouds
PRECIPITATIONF alling liquid or solid
TRANSPIRATION release of water vapor to atmosphere by plants
SUBLIMATION Require Energy, Conversion of a solid directly to a gas
DEPOSITION Release Energy, Conversion of a gas directly to a solid, without passing through an intermediate liquid phase
Humidity The general term used to describe the amount of H₂O(g) in the air
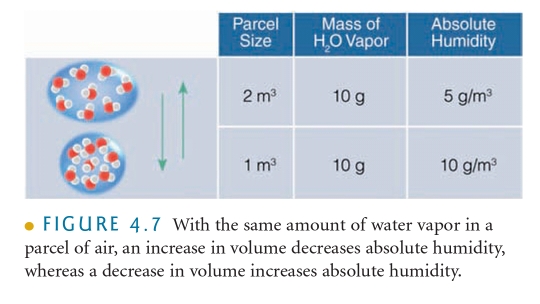
AH The MASS of water vapor in VOLUME of air
SH The MASS of water vapor in a unit of air compared to the remaining MASS of air (include water vapor) molecules
MR The MASS of water vapor in a unit of air compared to the remaining MASS of dry air
VP Total atm P attributable to its H₂O content
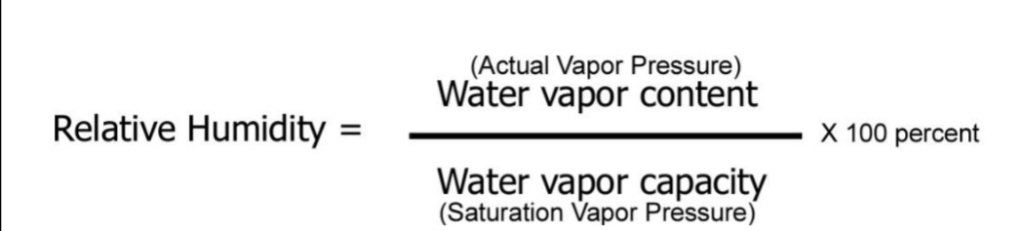
RH the ratio of the amount of H₂O actually in the air to the maximum amount of H₂O required for saturation at that particular T & P, or, ratio of the air’s H₂O content to its capacity
Dew Point T temperature which air needs to be cooled to reach saturation, measure of actual moisture content in air
Dew The condensation of water vapor on objects that have cooled to the dew-point
Frost NOT frozen dew, forms from deposition
Sensible T T we perceive In cold weather, when the air is calm, higher than a thermometer might indicate
wet-bulb T good measure of how cool the skin can become is the “” the lowest T that can be reached by evaporating water into air
HI Index combines air T with RH to determine an apparent T, what the air T “feels like” to the average, for various combination of air T & RH
Saturation VP When air is saturated VP exerted by motion of H₂O(g), Varies with T
Psychrometer Common instrument used to measuring RH, consists of 2 liquid in glass thermometers mounted side by side & attached to a piece of metal that has either handle or chain at one end
Hair Hygrometer measure RH, constructed on the principle “relative humidity increases, the length of hair increases”
Electrical hygrometer flat plate coated with film of C, electric current is sent across plate. As H₂O absorbed, the electrical resistance of the C changes & translated into RH
Infrared Hygrometer Measures RH by amount of IR absorbed by H₂O, & the dew cell determines the amount of H₂O in the air by measuring the air’s actual H₂O pressure
The End
Online Quiz
Seasonal & Daily Temperatures
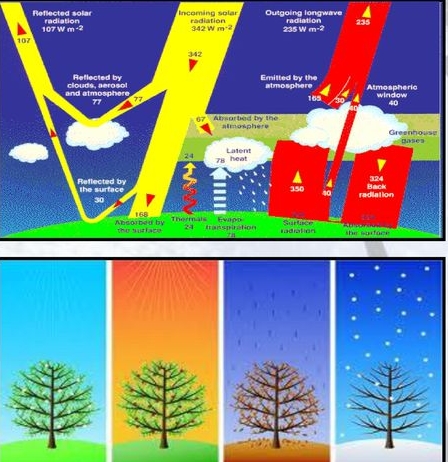
Important Terms
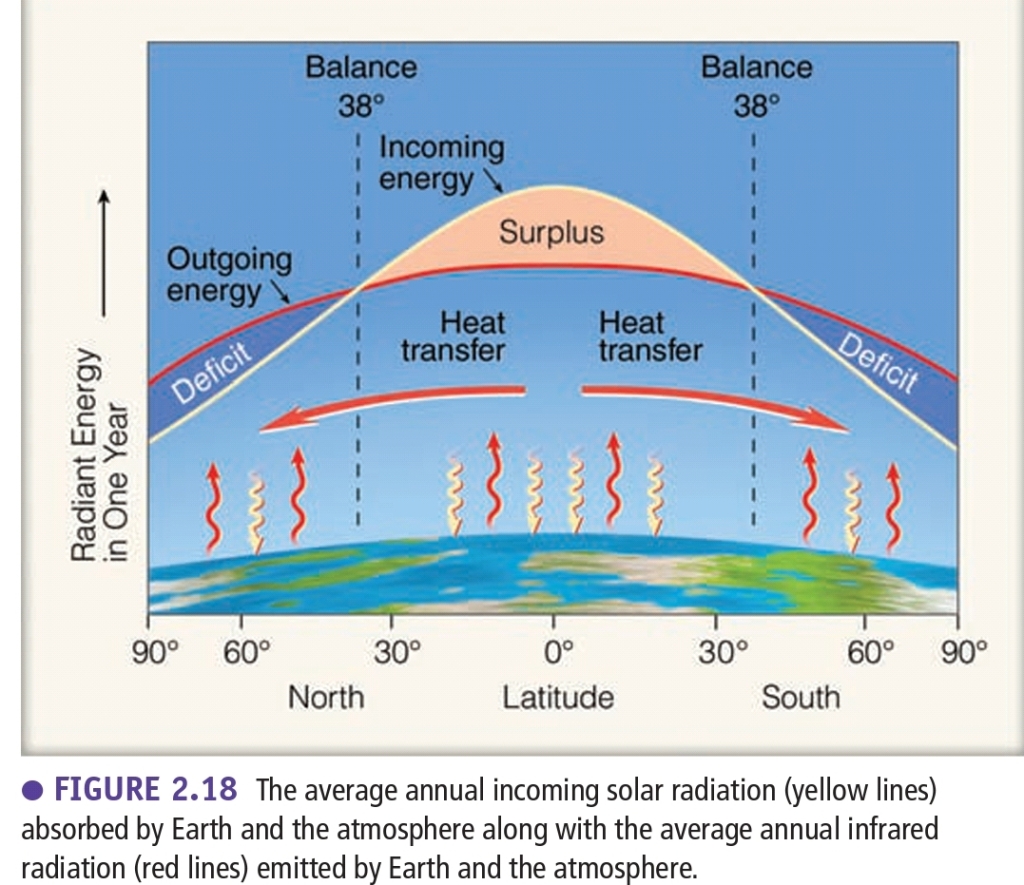
Circle of Illumination: Boundary separating the dark & light halves of the Earth
Earth’s Axis: Imaginary lines through the poles with Inclination 23.5°
Plane of the ecliptic: the plane of the orbit around the sun
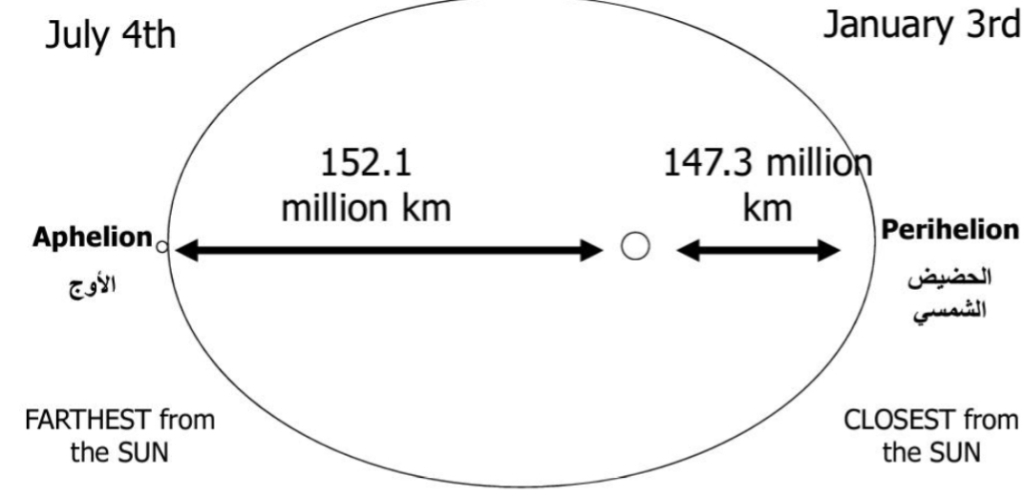
The distance between the Earth & the sun
average distance = 150 million km
Because earth orbital is ellipse the actual distance varies during year:
– in January 147 MKm, closer to the sun
– in July 152 MKm, Farther from the sun
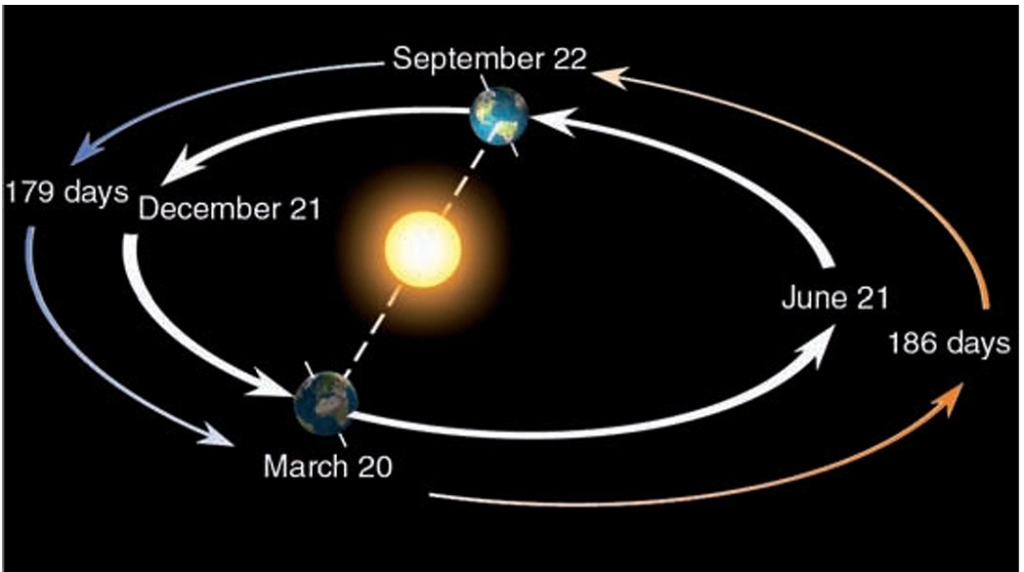
The Earth has 2 principle motions
Rotation: spinning of Earth about its axis
Revolution: The earth revolves completely around the sun in an elliptical path in 365 days, Travels at nearly 113,000 km/hr
(Elliptical Orbit – Eccentricity ≈ 0.8)
Τhe Seasons
Variations distance DO NOT cause seasonal temperature change
Without the TILT we wouldn’t have seasons
The Seasons primarily due to:
1. Change in the length of daylight
2. Gradual change in angle of the sun at noon
3. amount of energy received at surface
The seasons are regulated by energy received at the surface that determined by:
1. Angle of sunlight that strikes surface:
– Overhead, perpendicularly, or directly: is more intense (or strongest intense)
– at Angle (θ) is less intense
2. how long sun shines (daylight hours): determine how warm the surface becomes
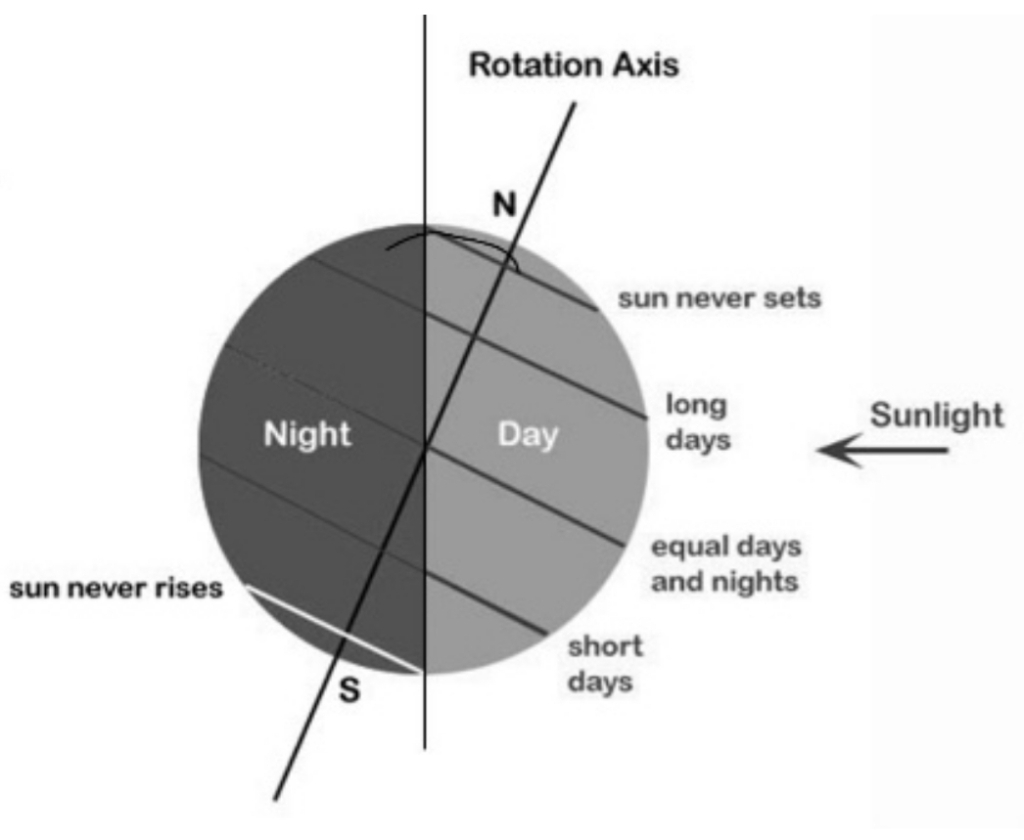
The change in day length & sun angle happens because: earth’s Rotation (earth’s orientation to sun constantly changes)
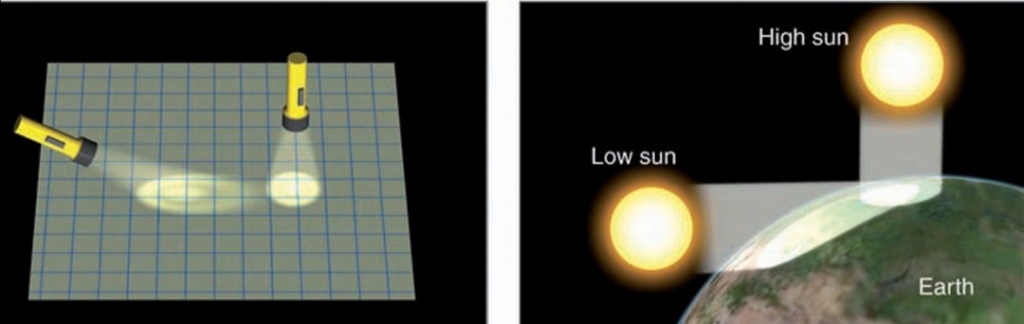
Length of Daylight & Solar Energy
During the summer in N latitudes, the sun is never very high above the horizon, so its radiant energy must pass through atm, & because of the increased cloud cover during the arctic S, much of the sunlight reflected before it reaches ground
Solar energy eventually reaches the surface in the N does not heat the surface effectively
A portion of the sun’s energy is reflected by ice & snow, while some of it melts frozen soil
The amount actually absorbed is spread over a large area. So, even though N cities experience 24 hr of continuous sunlight on June 21, they are not warmer than cities S
Overall, they receive less radiation at the surface, & what radiation they do receive does not effectively heat the surface.
The Earth intercepts only a tiny percentage of the energy given off by the sun
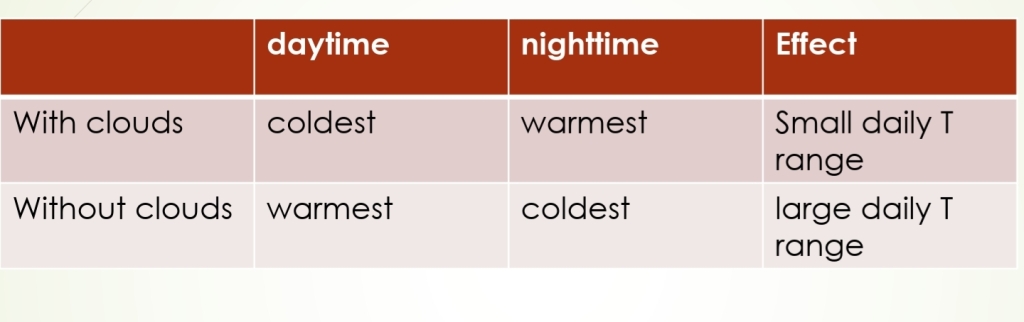
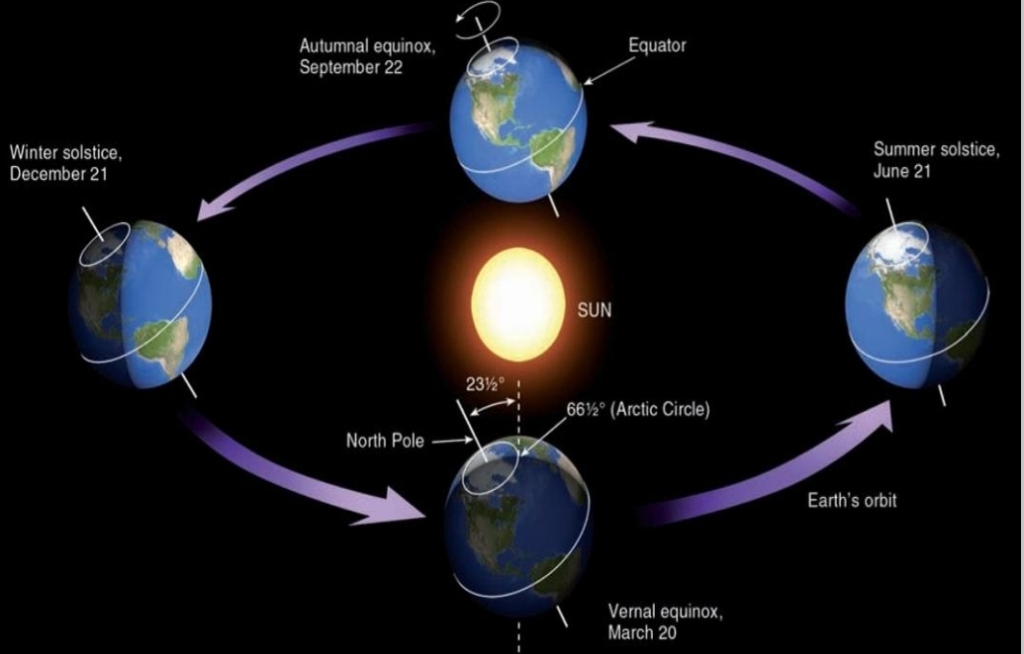
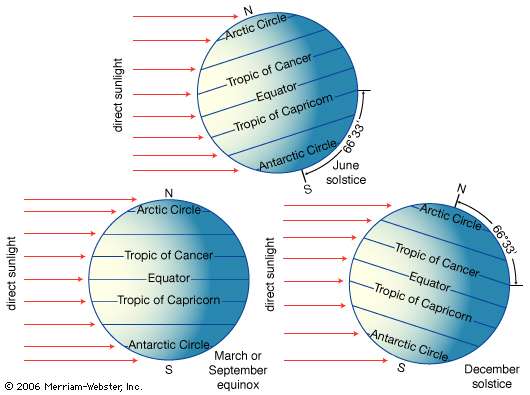
Summer Solstice
– Occurs on June 21 or 22
– Tropic of CANCER (23.5°N latitud)
– Northern limit of the Sun’s rays
– First day of NH summer
– NH tilted toward the sun
– NH location experience LONGEST day
– ΝH location experience HIGHEST Sun θ
– SH location experience SHORTEST day
– SH location experience LOWEST Sun θ
– Farther from the equator → longer period of daylight (Arctic Circle has 24hr of SUN)
Winter Solstice
– Occurs on December 21 or 22
– Tropic of CAPRICORN (23.5° S)
– Southern limit of the Sun’s rays
– First day of NH winter
– NH tilted away from Sun
– NH location experience SHORTEST day
– ΝH location experience LOWEST Sun θ
– SH location experience LONGEST day
– SH location experience HIGHEST Sun θ
– Farther from the equator → longer period of daylight (Antarctic Circle has 24hr of SUN)
Equinoxes
mid way between the Solstices
– Vertical rays strike along the equator 0°
– Earth not tilted toward or away
– Autumnal Equinox: September 22 or 23
– Spring(Vernal) Equinox: March 21 or 22

Tilt
More Tilt (> 23.5°): More extreme seasons, Colder winters, & Warmer summers
Less Tilt (<23.5): More mild seasons Cooler summers, & Milder winters
Tilt = 0 (NO TILT): No Seasons
– The location of Tropic of Cancer & Capricorn shift (Ex. 30° Tilt: tropic of Cancer 30°N & tropic of Capricorn 30°S )
– The Arctic & Antarctic circles shift 90-θ (Ex. 30° Tilt: Arctic Circle 60°N & Antarctic 60°S)
Air T is important, Why ?
It’s the first thing we usually think when we talk about “weather”
vary on different time scales: Seasonally, daily, & hourly
vary all over the globe, by quite bit
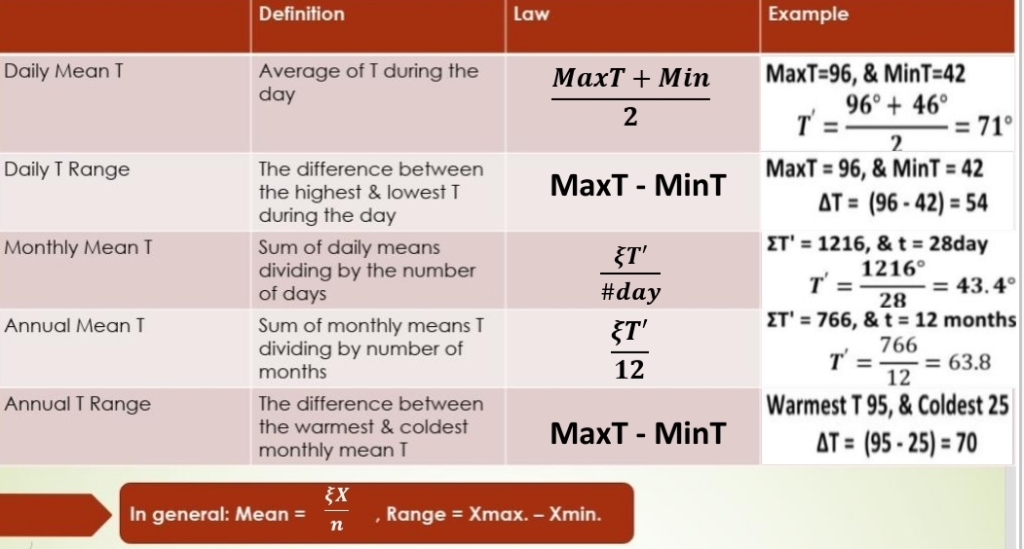
Day Vs Night
1. On a sunny day, the air near the surface warmer than air above the surface
2. On a night, the air near the surface is colder than air above the surface
Temperature Inversion: The increase in T with increasing height above the surface (on a night)
Radiational Cooling: Radiation Inversion, Nocturnal inversions
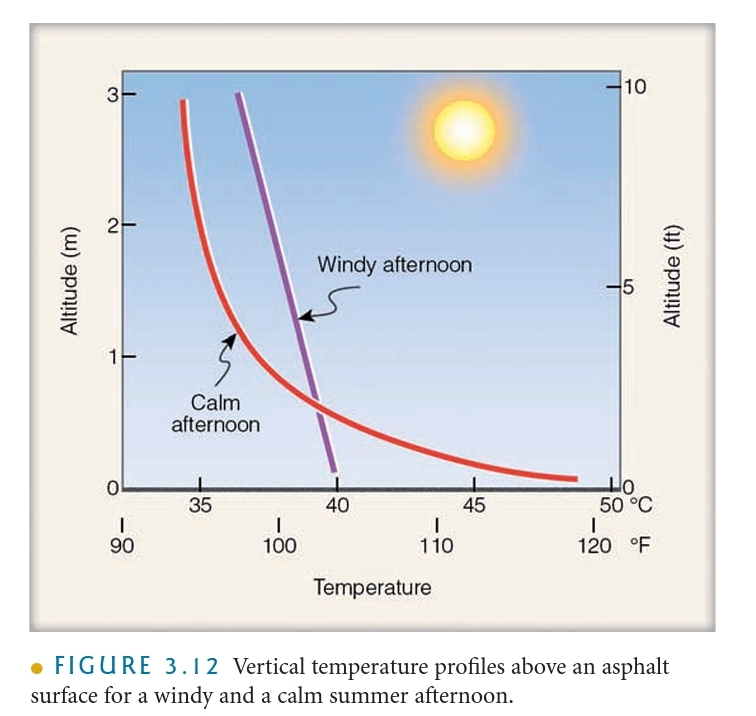
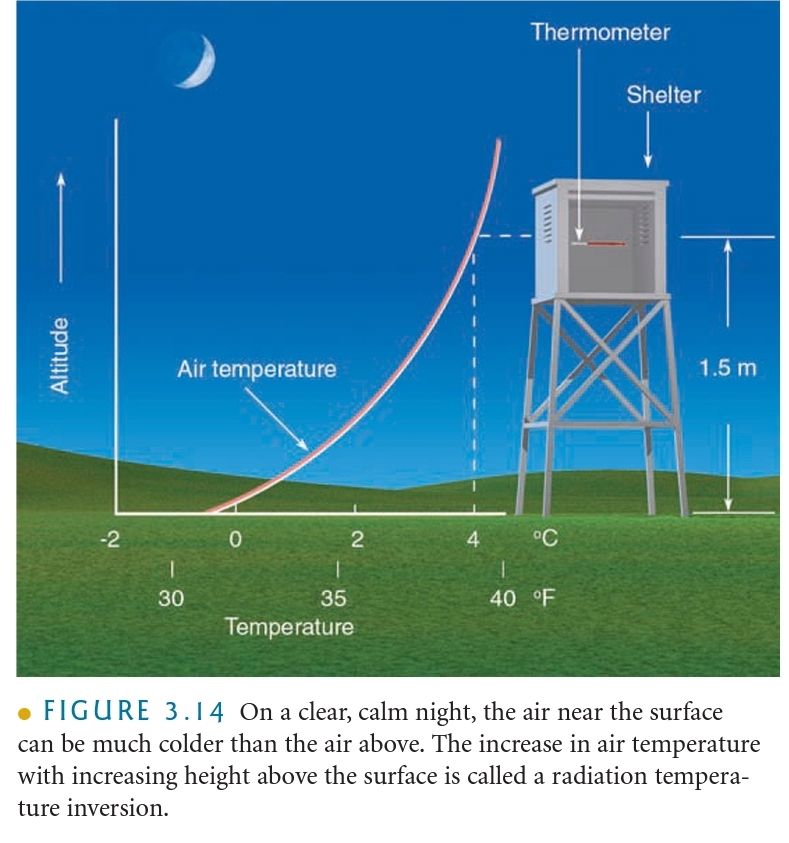
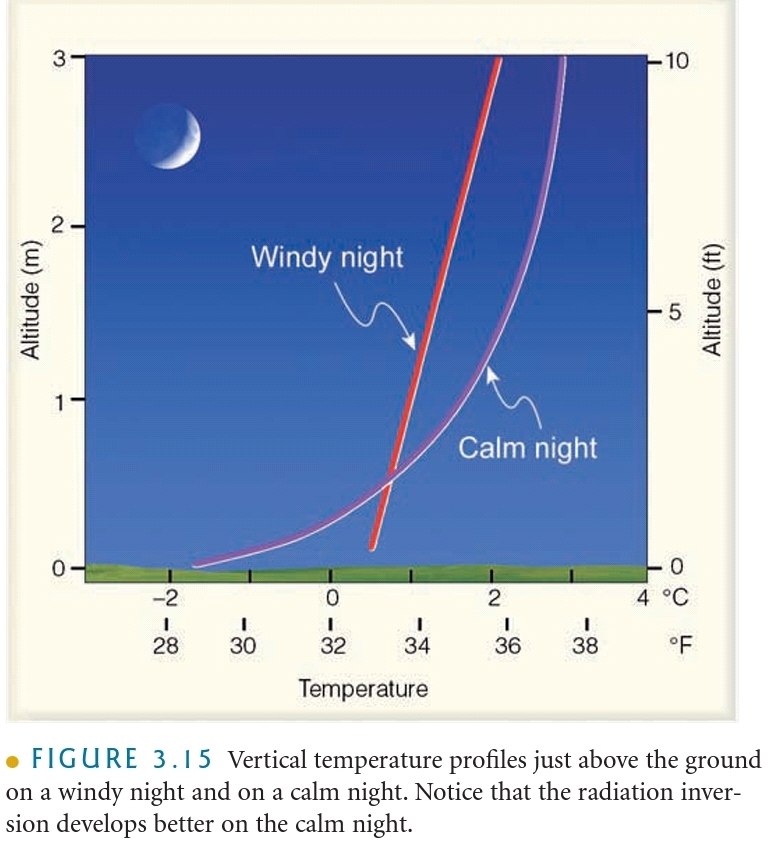

What controls air temperature?
Differential Heating of Land & Water
Ocean Currents
Altitude
Geographic Position
Cloud Cover
Albedo
Differential Heating
Different surfaces absorb, emit, & reflect different amounts of energy, & This causes variations in air above each surface
Land HEATS or COOLS more rapidly so Variations over Land are GREATER
Ocean less variable, Why?
1. Surface T of water rises & falls slower
2. Water is highly mobile & mixes easily
3. Daily T changes are about 6m deep
4. Yearly ocean & deep lakes experience variations through layer (200-660)m thick
Land are more variable, Why?
1. Heat not penetrate deeply into soil or rock & it remains near the surface
2. Rocks are not fluid… so no mixing
3. Daily T changes are 10cm down
4. Yearly T changes reach < 15m
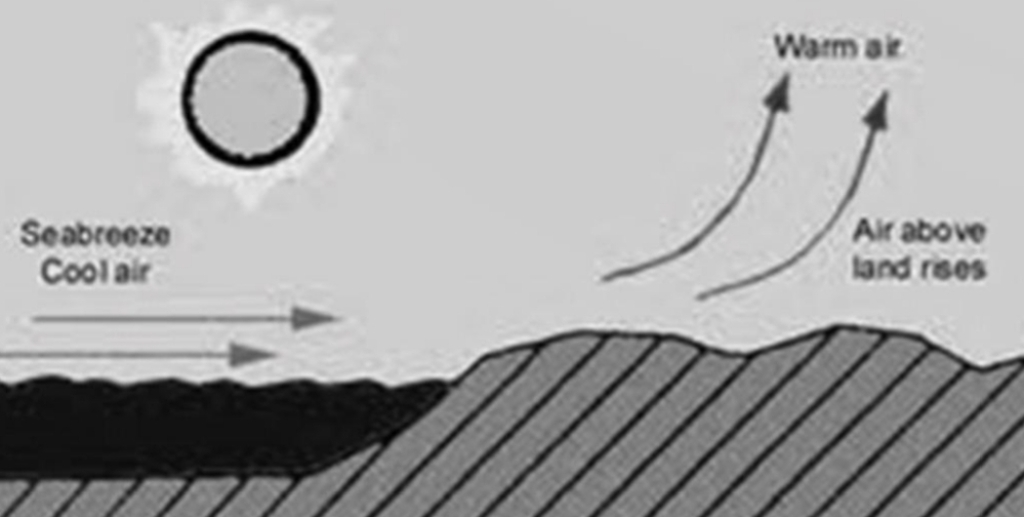
Summer Vs Winter
During summer thick layer of water is heated while only a thin layer of land is heated
During winter the shallow layer of rock cools rapidly while the deeply heated water takes a longer time to cool
as surface water cools, becomes heavier & sinks, replaced with warmer less dense water from below, So water surface T not appear to change much
Opaque Vs Transparent
land surfaces are opaque, So heat is absorbed only at the surface
Water is transparent & lets energy from the sun penetrate to a depth of several meters
Specific Heat
amount of heat needed to raise T of 1g H₂O by 1°C (3 times greater than 1g of soil or rock)
The OCEANS require More Reheat to raise its T same amount as an equal quantity of land
Evaporation
greater from Oceans than from Land Because There’s more water molecules (Intuitive!)
Energy is required to evaporate water: When energy is used to evaporate water it is not available for heating
WATER WARMS MORE SLOWLY THAN LAND!
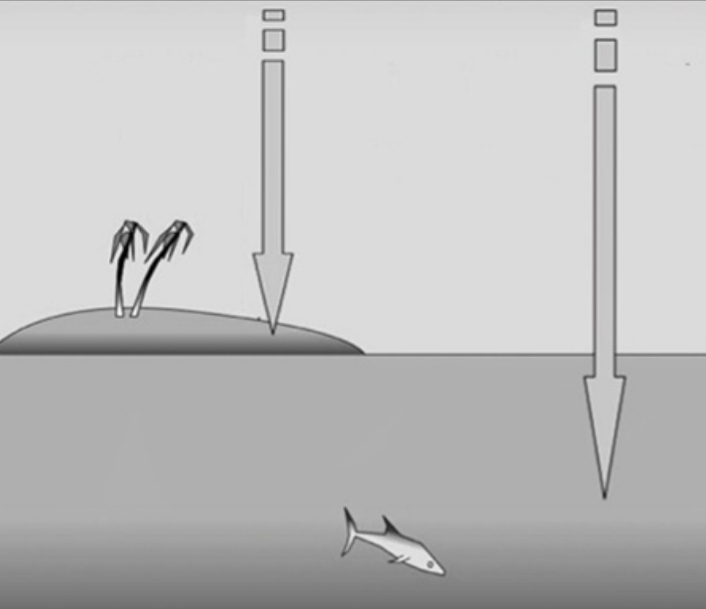
Which Hemisphere (N or S) has larger T variations? & Why?
NH, there is more ocean & little land to interrupt the oceanic & atm circulation
THE END
Online Quiz
Learning
Temperature & Heat Transfer
Important terms
Energy ability or capacity to do work, result from motion such as kinetic energy
Matter anything has mass & occupies space
Kietic energy (k) energy from motion
Calorie: amount of heat required to raise T of 1gH₂O from 14.6°C to 15.5°C (1cal = 4.186J)
kilocalorie: is 1000 calories, heat required to raise 1kgH₂O 18°C
Heat capacity: ratio of heat absorbed by substance to its corresponding T rise
C = Ε/ΔΤ [J/°C]
specific heat: heat capacity of a substance per unit mass, or amount of heat needed to raise the T of 1g of a substance 1°C(Ex. If heat 1g of water on a stove, it take 1cal to raise its T 1°C)
S = C/m = E/mΔΤ [J/g°C]
Sensible heat: the heat we can feel, “sense,” & measure with a thermometer
Important concepts
Work is done on matter if pushed, pulled, or lifted over distance
The energy within a body result of its motion, such as Potential & Kinetic energy
Temperature
average of Kinetic energy or average speed of atoms & molecules (energy within body that result of its motion)
describes how warm or cold an object is

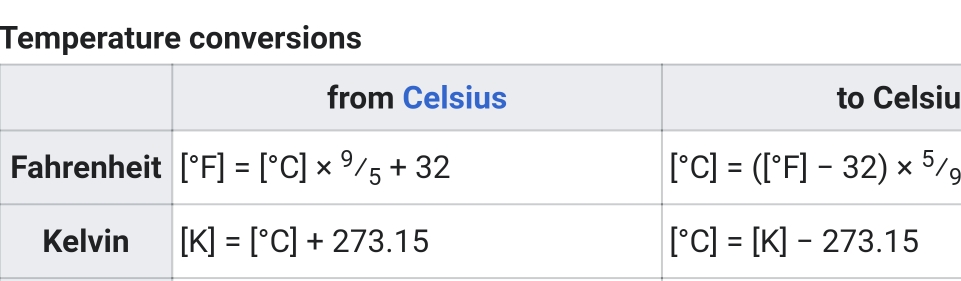
Heat
ENERGY that TRANSFER from one body to another due to Differences in temperature
FLOW OF ENERGY due to Differences in T
After energy transferred it stored as internal energy
Latent Heat
energy required to change state of substance
Ex. H₂O(s) –> H₂O(l), T in these reaction stay constant & Heat is used to MELT the ice doesn’t produce a T change
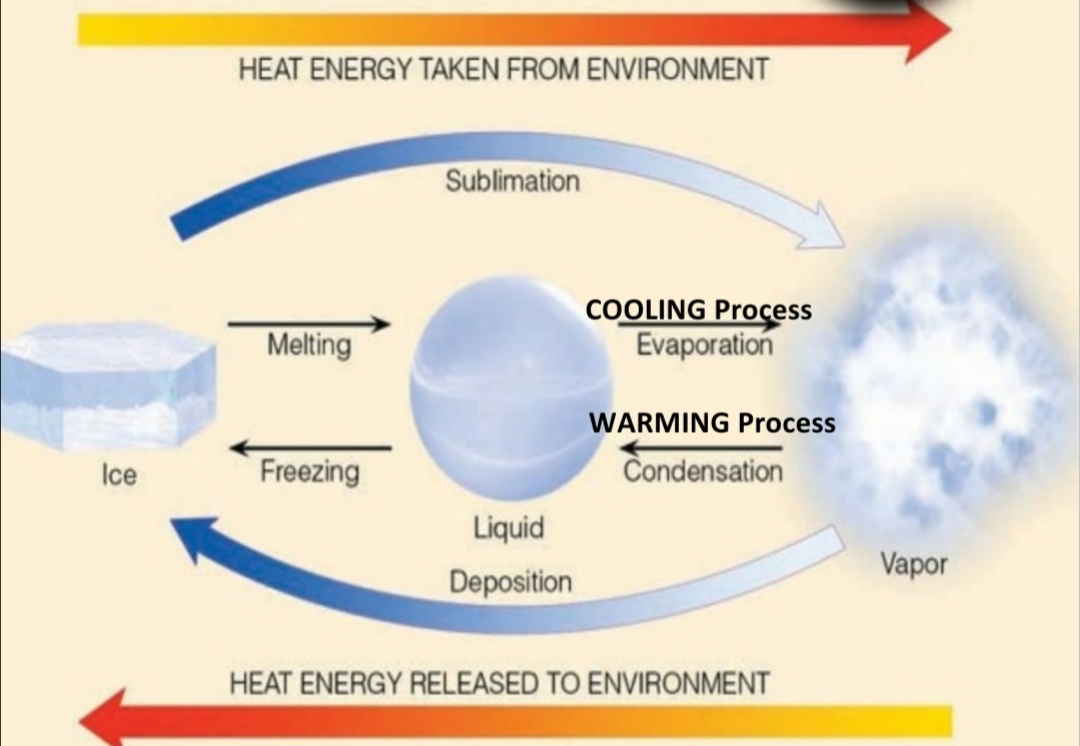
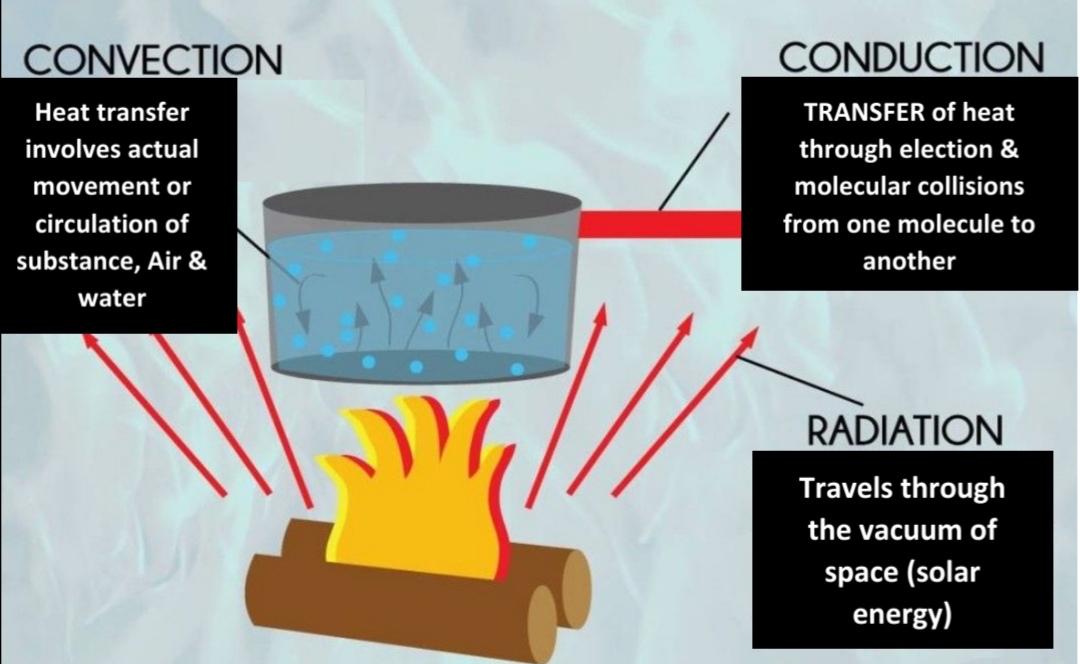
Ability to conduct are varies
Metals are better
Air INSULATOR (not conduct well)
Convection
Most common in atm (Only important for heating air in DIRECT contact with surface)
Thermals: circulation movement
Advection: horizontal movement

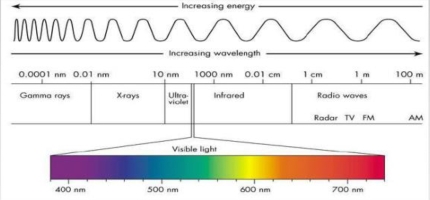
Wavelengths (λ)
Is a distance between 2 crest
All λ travel at 300,000 km/s
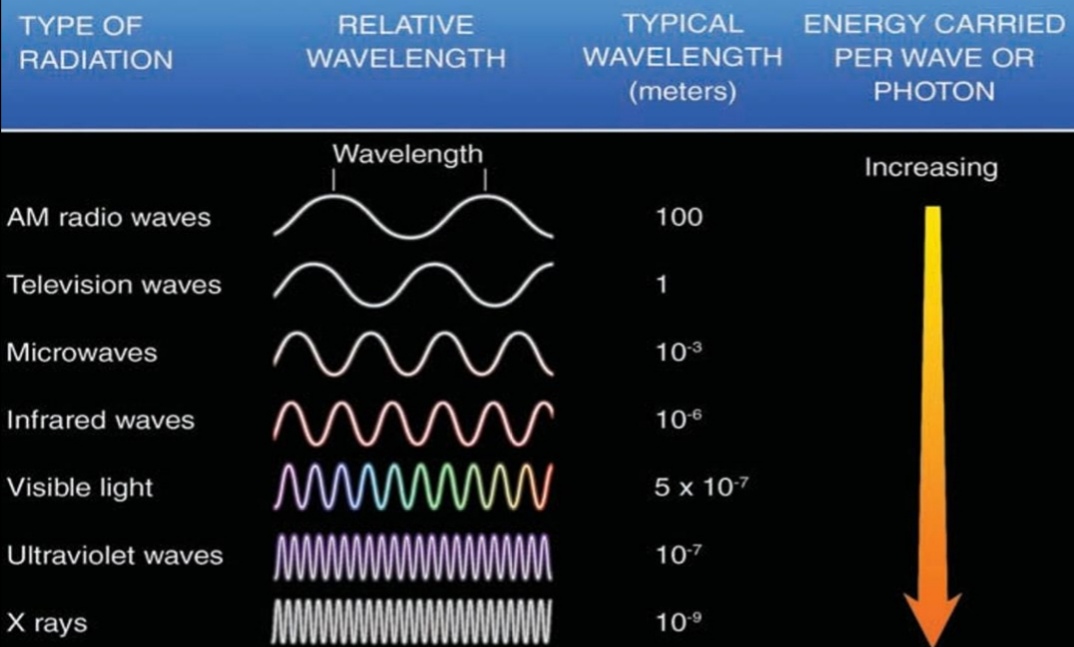
Radiation emitted by the Earth
Earth emits radiation at longer λ than the sun (Emits less Energy)
> 95% of Earth radiation λ = (2.5, 30)μm (IR)
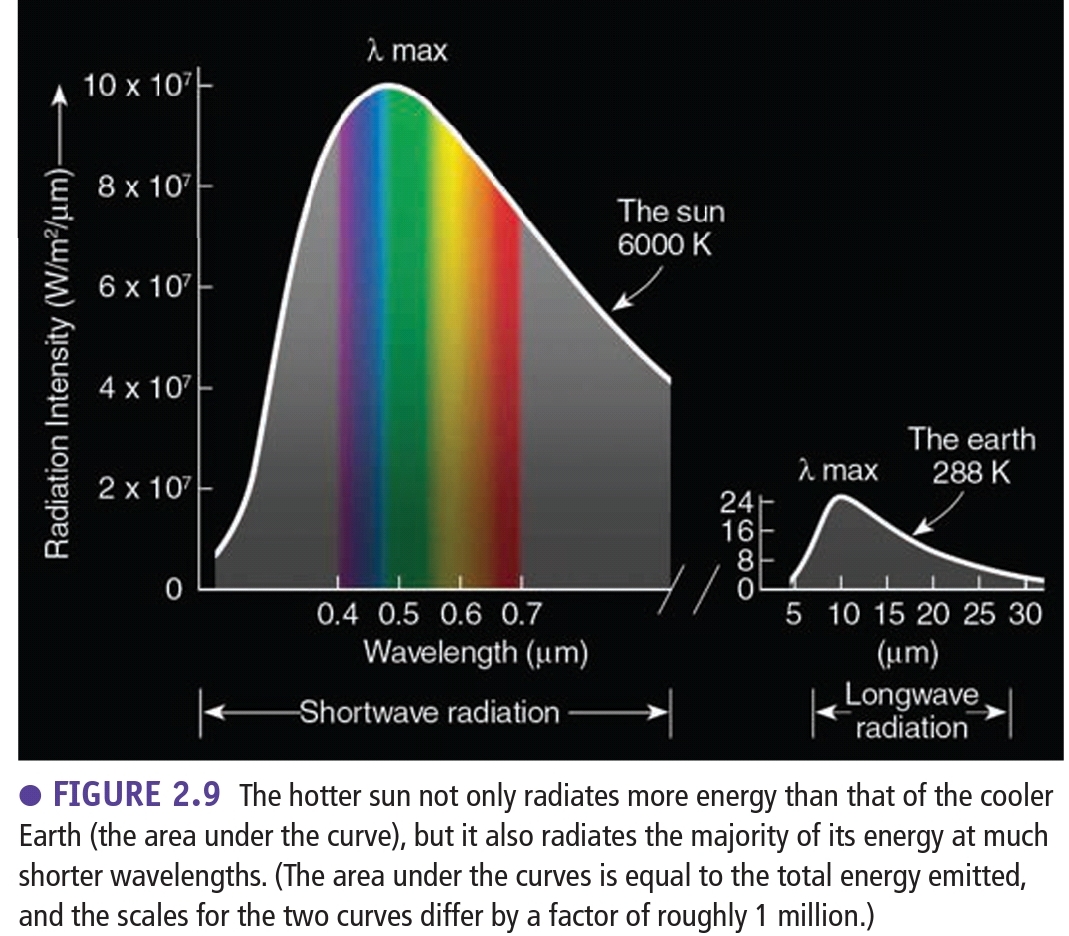
Laws of rediation
All objects emit radiant energy over range of λ (EVERYTHING emits energy), Unless it’s at “absolute 0” if molecules stop
Hotter objects radiate more energy in the form of short λ than cooler objects
Stephan-Boltzman Law: Hotter objects radiate more total E per unit area
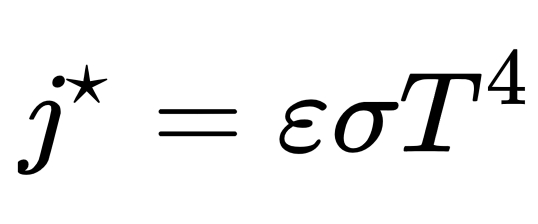
Blackbodies
such as Sun & Earth
Earth’s radiative equilibrium T = 255°k = -18°C = 0°F
Why isn’t this average surface T? atm IS NOT black body (Gases selective absorbers, absorb, & emit IR)


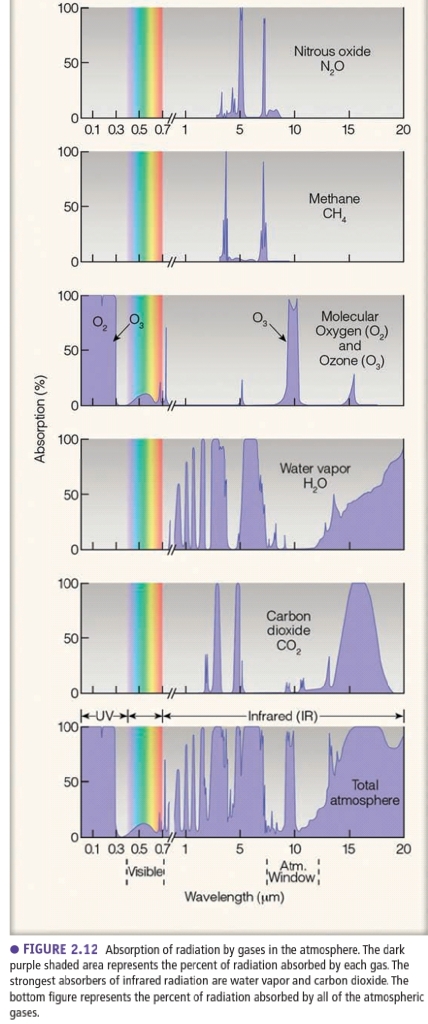
Gases are most effective absorbers of radiation & play primary role in heating atm
1. H₂O, O₂, O₃ absorb most of energy in atm
2. CO₂ is important at long λ (IR)
3. “Openings” are atmospherec windows

Atmosphere warms planet
H₂O, CO₂ (called selective absorber) absorb outgoing IR & absorbed energy heating the air
GHG
The Earth’s average T = 33°C = 59°F
GHG “villain” in Global Warming Debate
GH Effect & Global Warming NOT same thing
Without GH Earth would be uninhabitable!
Human activity may be making atm more efficient at retaining long wave emissions from the Earth
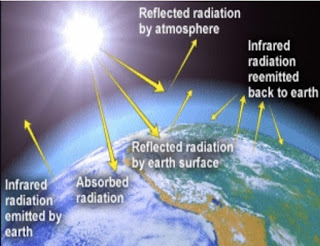
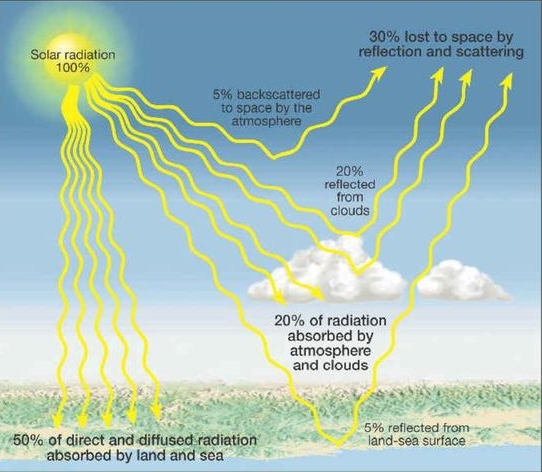
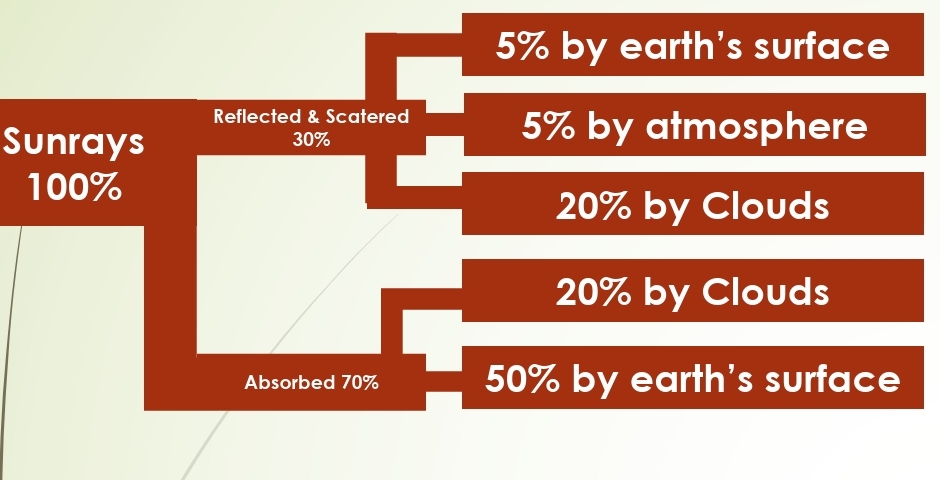
What Happens to Incoming Radiation?
Absorbed, Transmitted, Reflected, Scattered
DEPENDS ON λ
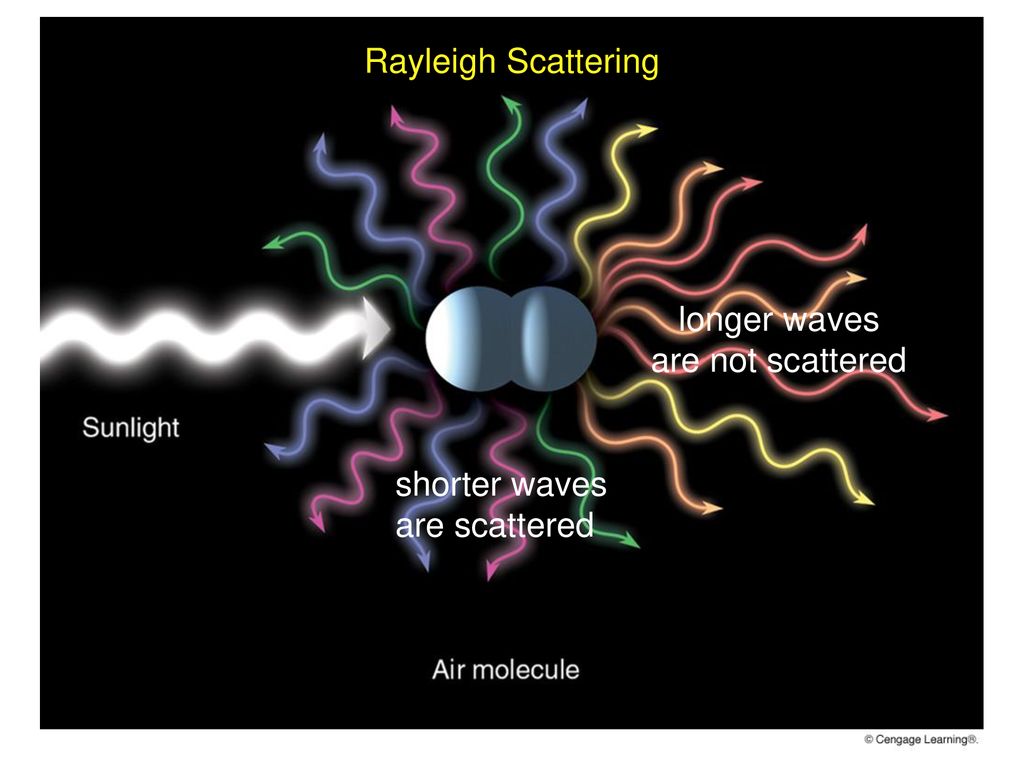

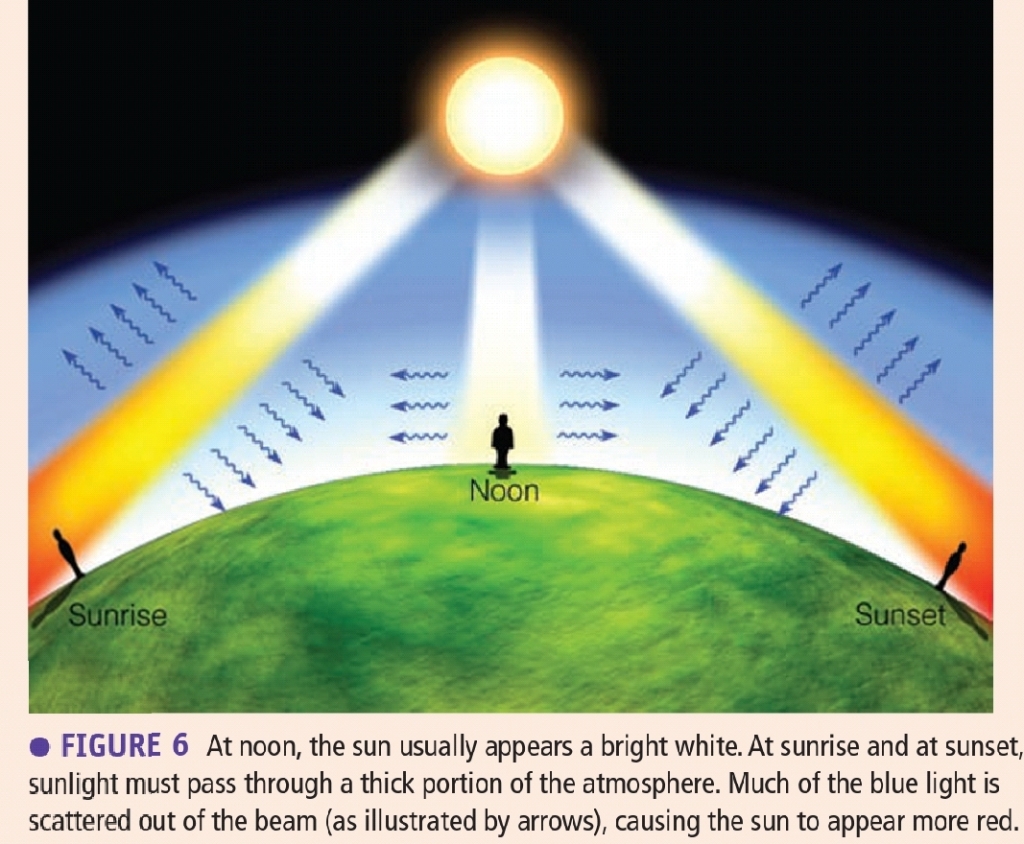
Scattering
produces large number of weaker rays
Air molecules tend to selectively scatter
Blue Skies, Red Suns, & White Clouds duo to scattering
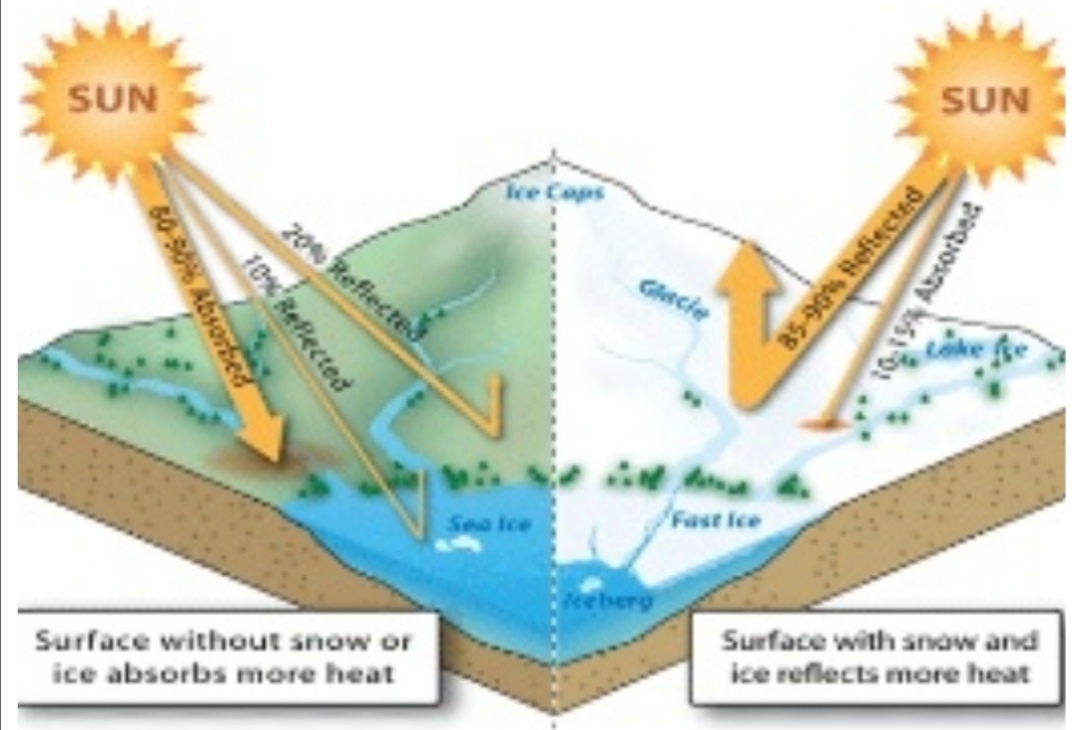
Reflection
bounces off at same angle & intensity
Reflection & Albedo: Energy is returned to space via reflection & emission
ALBEDO: percentage reflected (30%)
5% from land & ocean
25% from clouds & ice

Clouds absorbers IR radiation & effect of heating the earth depends on type of cloud
The effect of heating depends on type of cloud
1. Thick cloud absorbs the most of outgoing IR, & re-radiating it back to the surface (Warm cloudy nights)
2. High Thin Clouds Tend to WARM the surface by transmit incoming SW & Absorb outgoing LW & re-emit it back down
3. Low Thick Clouds Tend to COOL the surface by block incoming SW, have high albedo so reflect SW back to space
On average: clouds Cool the Earth
Note. SW = Short Wave, LW = long wave

T decrease in troposphere, Why
Surface warms the traposphere (The atm is HEATED from the GROUND UP)
Whether specific clouds will warm or cool surface depends on
1. time of day
2. Cloud’s thickness & height above surface
3. Surface with Liquid, dry Land, or ice
Earth’s average T constant due to
balance of incoming & outgoing radiation (black body)
Seasons-Regulated by
amount of solar energy received by surface
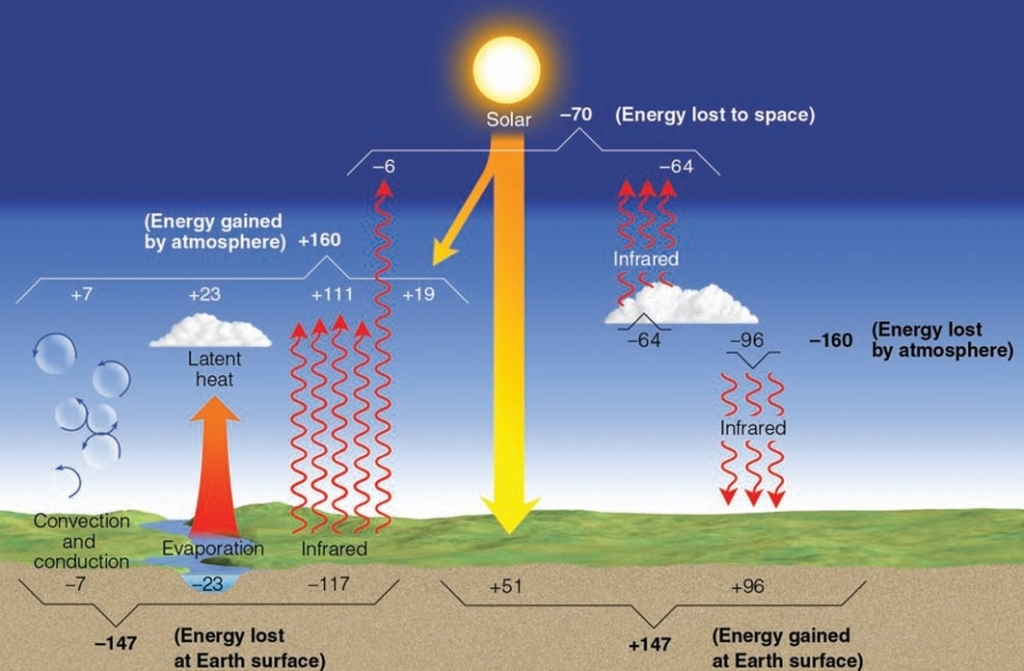
Why don’t tropics keep getting hotter & poles colder?
Movement of atm & oceans transfer energy from the equator to the poles
energy imbalance (unequal heat) drives ocean currents & winds (Weather)
The End
Online Quiz
Learning
INTRODUTION
الارض، مُقدمة
بدأ تاريخ الأرض والمجموعة الشمسية بسحابة سديمية Primordial Nebula من غازات وغبار كوني
نشأ بمركز السدیم دوامة كبيرة أدت لتدافع مادته نحو داخله فيما يعرف بالانكماش الجذبي Gravitational Attraction
استمر الانكماش فازداد الضغط والحرارة بجوفه فأدى لبدء تفاعلات نووية أشعلت الهيدروجين فأضاءت الشمس التي كانت خاضعة لقوتين متعادلتين هما الطاقة الحرارية من جوفها التي تزيد حجمها والانكماش الجذبي الذي يقلص حجمها فاستقرت حتى يومنا هذا
حدثت دوامات صغيرة بأطراف السديم أدت لنشوء الكواكب التسعة المحيطة بالشمس ونظرا لصغر حجم السديم لم تصل درجة حرارة جوفها لدرجة بدء التفاعلات النووية فبقيت الكواكب أجسامة باردة غير مضيئة
الأرض والكواكب القريبة من الشمس خضعت اثناء تكاثف سديمها للرياح الشمسية التي طردت الغازات المكونة للسدیم وبقيت العناصر الأكثر ثقلا وكذلك دقائق الغبار الكوني التي تجمعت فشكلت الأرض الصلبة
جاءت مرحلة النشاط البركاني الشديد التي دفعت كميات كبيرة من الغازات لجو الارض اهمها بخار الماء الذي هطل مكونا مياه البحار واصبح هواء الارض مكونا من غازات مثل كبريتيد الهيدروجين والامونيا والارغون والهيليوم والنيتروجين وغيرها،
الاكسجين لم يكن موجود فكان الجو مختزل Reducing
كانت الأشعة فوق البفسجية تصل لسطح الارض اذ لم يكن بغلاف الأرض ما يمنعها من الوصول وقد فككت او تفاعلت هذه الاشعة عالية الطاقة مع بعض اكاسيد الغازات كبخار الماء مثلا محررة الأكسجين غير أن هذا الأكسجين الحر كان يستهلك بسرعة من الغازات المختزلة، سميت هذه المرحلة مرحلة التمثيل الكيميائي chemosynthesis
قبل 3500 مليون سنة بدأت كائنات حية كالبكتيريا بالوجود على الأرض والتي لا تحتاج الأكسجين بل تاخذه من عمليات كيميائية ، غير أن اهم ماكانت تقوم به انها كانت تاخذ ثاني اكسيد الكربون من الجو لتصنع غذائها بعملية التمثيل الضوئي Photosynthesis
كانت هذه الكائنات تطلق الأكسجين الحر الذي ادي لظهور الكائنات الحية التي تحتاج الأكسجين
الأرض شهدت عمليات جيولوجية نتج عنها تكوين المعادن كالحديد والفضة والذهب والرصاص والماس والفوسفات وتكونت مصادر طاقة احفورية كبترول وغاز وفحم حجري
More than 150 people die each year in USA from floods & flash floods, more than any other natural disaster
Why do we study Meteorology?
1. Daily Weather: how we plan our days
2. Severe Weather: damage, & loss of life
3. Climate Change: quality of life, water & food supplies
Severe Weather Includes
tornadoes, hurricanes, snow storms, floods, thunderstorms…etc
Recent Weather Events
1. Hurricane Sandy: 2012, > 253 people killed in 7 countries, Cost estimated at 65.6B$, which would make it the 2nd costliest Atlantic Hurricane behind only Katrina.
2. Joplin Tornado: 2011, 158 people killed & > 1,100 injured, It was dead lies tornado in America since 1947
3.VOG:2012, Trade Wind Weaken (respiratory complaints, headache, watery eyes, severe sinus infections), sulfur dioxide SO₂ gas & sulfate SO₄ aerosol which can cause acid rain/acid particles
4.Great Plains Drought: 2012, Worst hit region is the Central Great Plains, Critical for agriculture!
Meteorology
Is the study of atm & its phenomena
– mathematical science ❤
– The term goes back to Aristotle who wrote a book about meteorology
– Meteor means “high in the air”, Weather instruments in the 6th century
Weather
The state of the atm at any given time
Weather elements: Air T & P, Humidity, Clouds, Precipitation, Visibility, & Wind
Climate
sum of all statistical weather information (Average range of “weather element” over long period of time)
description of aggregate weather conditions
Includes extreme weather event (Droughts, heat wave, & cold snaps)
Climate changes on geological time scales such as ice ages
Climate VS Weather
Climate is what you expect, & weather is what you get
Evolution of the atm
1. First atm: H & He, 4.6 BY ago, swept away by solar winds, & escaping hot surface
2. Primeval Phase: mostly CO₂, N₂, H₂O, little CH₄, NH₃, SO₂, HCl
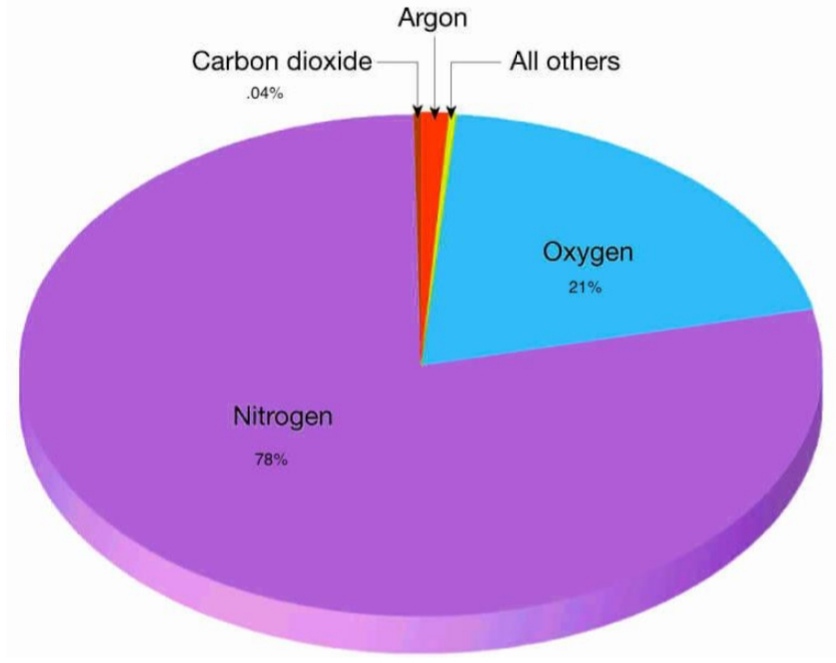
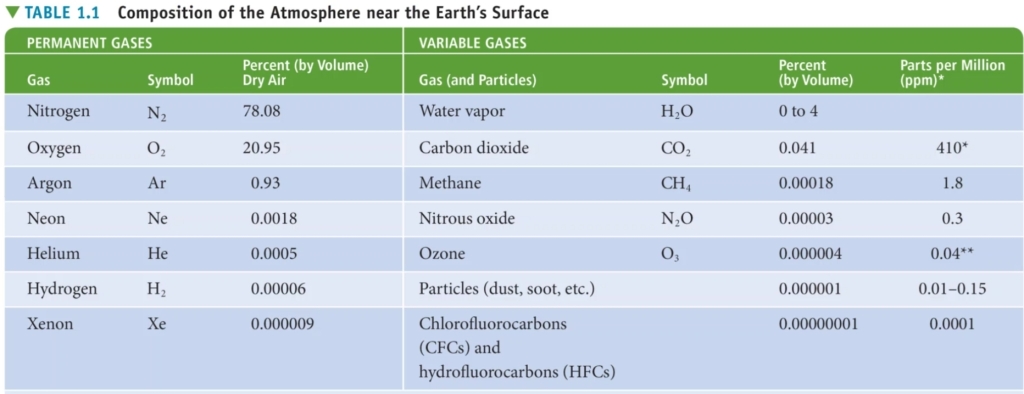
3. Μodern Phase: 78%N, 21%O, 1%Ar, <1% other gases & aerosols
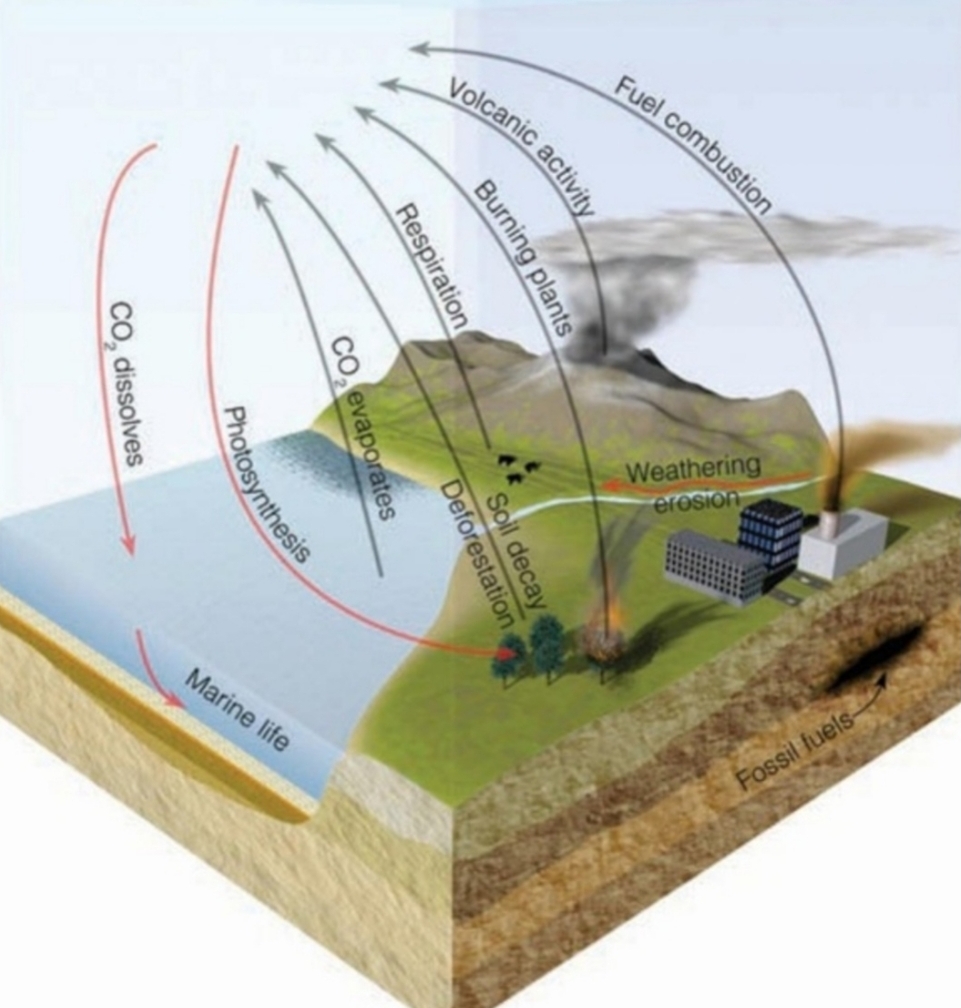
After 4 BY, surface cooled, Water vapor to condense into clouds so rain, Oceans, & rivers formed
CO₂ dissolves in H₂O: rain & ocean “washed out” CO₂, cooling the planet
O₂ building up in the atmosphere: After life emerged, photosynthetic bacteria emerged
Source of atm gases was OUTGASSING release from rock through volcanic eruptions & meteorites impact
Μodern Phase Composition
78% Nitrogen N₂
21% Oxygen O₂
< 1% Argon Ar
Carbo (–dioxide CO₂, -monoxide CO)
N (-dioxide NO₂, -oxide NO), Nitrous N₂O
Sulfer oxide SO, Hydrogen H₂, Helium He
Methan CH₄, & Ammonia NH₃
Ozone O₃, & Water vapor H₂O(g)
aerosols: tiny solid & liquid particle:
– dust, & smoke
– sea salt, & biogenic particle
– pollution, & volcanic ash
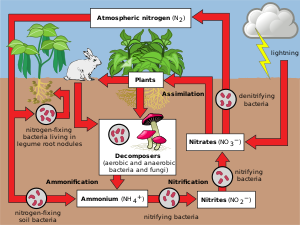
Where did all the Nitrogen come from?
1. N₂ is volatile in most of its forms
2. N₂ unreactive with materials that make up the solid earth
3. N₂ stable in solar radiation environment
– Over geological time, it has built up much greater than O₂
– It is an important component of life on earth (N-Cycle)
Why is Argon Third?
1. formed by radioactive decay of potassium isotope, & Released into atm by volcanic activity
2. inert gas, nonradioactive, so gradually accumulates in atm
-Used in Neon Lights
Where does oxygen come from?
1. Primary way is photosynthesis (98%)
2. The breakup of water molecules by ultraviolet radiation (1-2%)
Ozone O₃
concentrated above surface in stratosphere (good) & in Troposphere (poor)
Protect us from UV rays (what gives us sun burns)
Ozone Hole: in the Antarctic, though one happened in Arctic
Montreal Protocol

water vapor H₂O(g)
variable over the surface of the earth (0-4% by volume)
most abundant GHG: Heats atm (Releases or absorbs energy if it changes states )
Methan CH₄
GHG
from cows, wetlands, & rice pattie
low oxygen environment
Carbon dioxide CO₂
GHG
from respiration, combustion, & evaporation
Global Climate Change
present in tiny amouont ≈ 0.0387% ≈ 387ppm (increase since 1960s)
an efficient absorber of energy emitted by the sun!
atm Provides
air we breath
protection from damaging of UV radiation
as altitude (height) increases
layer Rapidly thins (density decreases)
P decreases (Not at a constant rate)
near surface air more dense, & Decreases rapidly at first then more slowly
P decreases as altitude increases
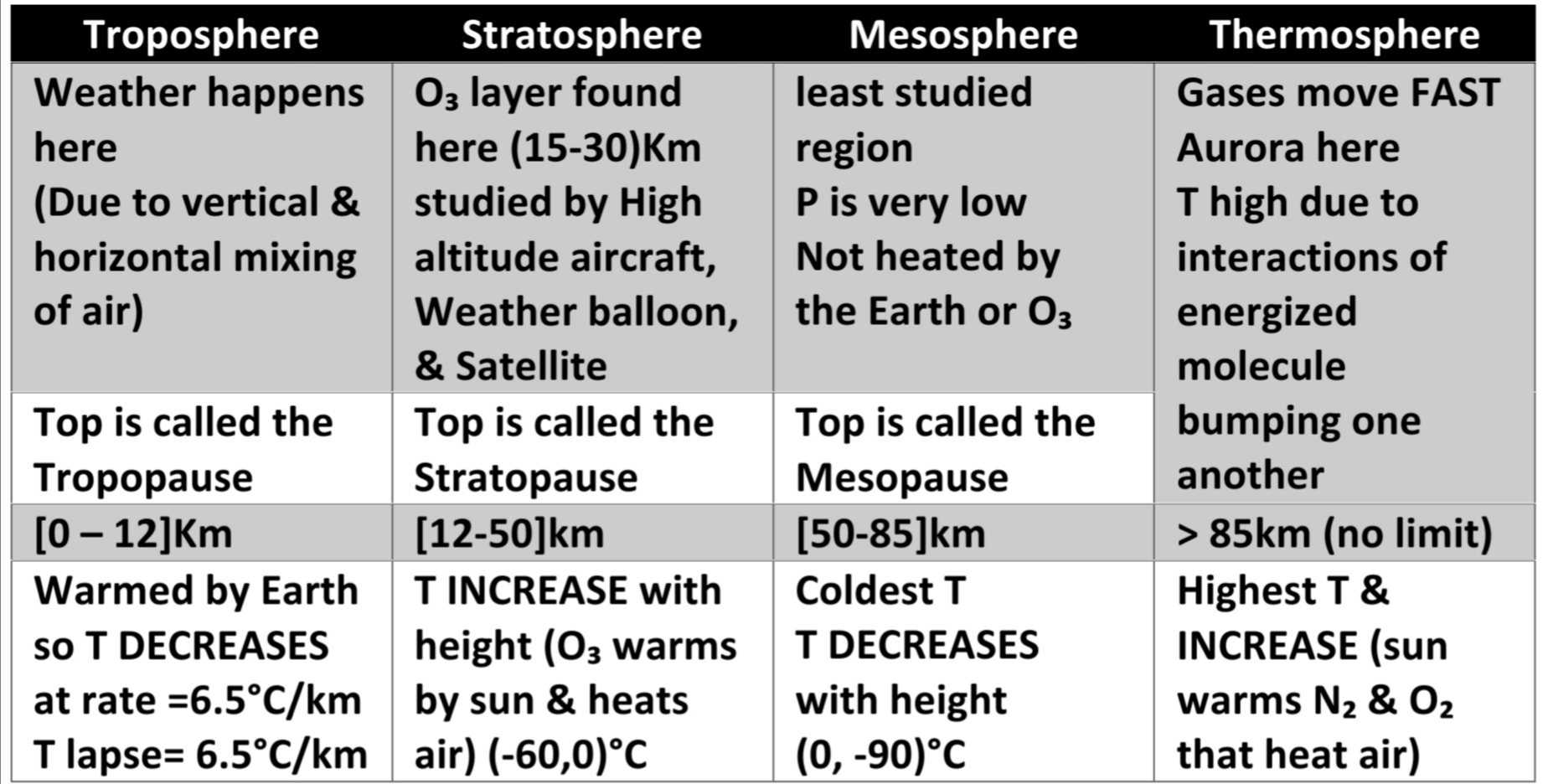
Layers of atm VERY THIN: 99% is within 30km
Half atm is lies below 5.6km
Standard atm P = 1013.25mb = 1013.25hPa = 29.92Hg
Air is HIGHLY compressible

What happen if astronaut exposed his hand in Thermosphere?
not feel hot, not enough particles
how the current atm evolved?
1. Outgassing
2. Photosynthesis
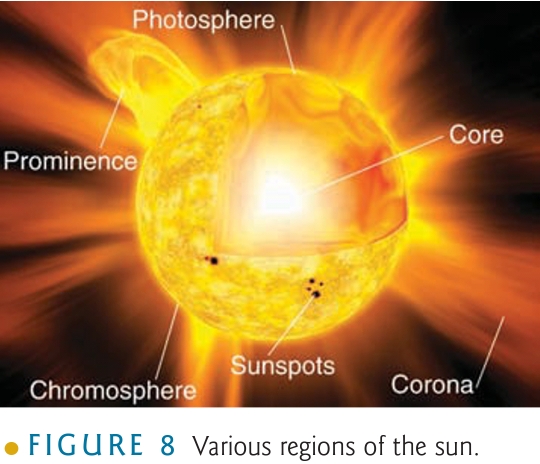
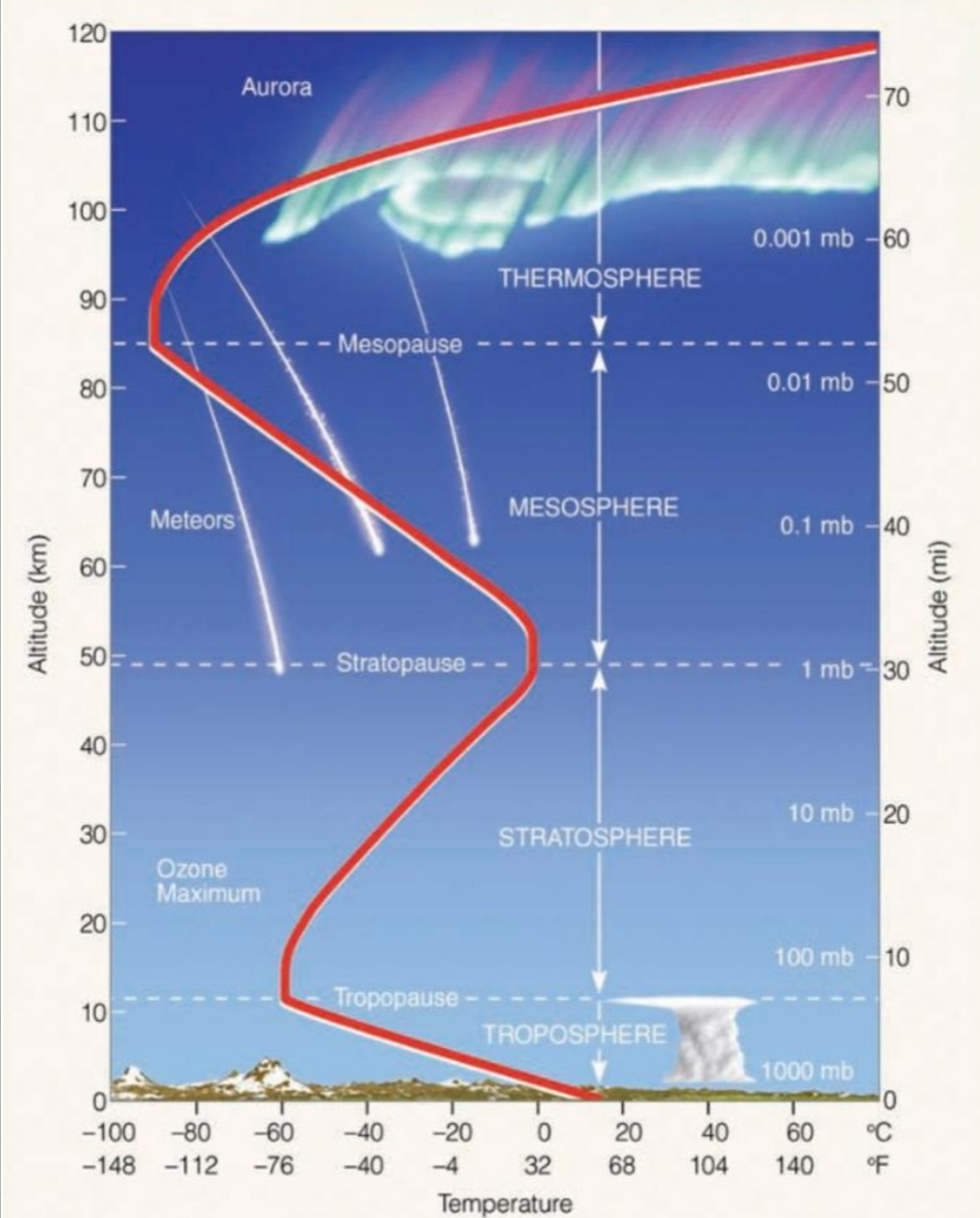
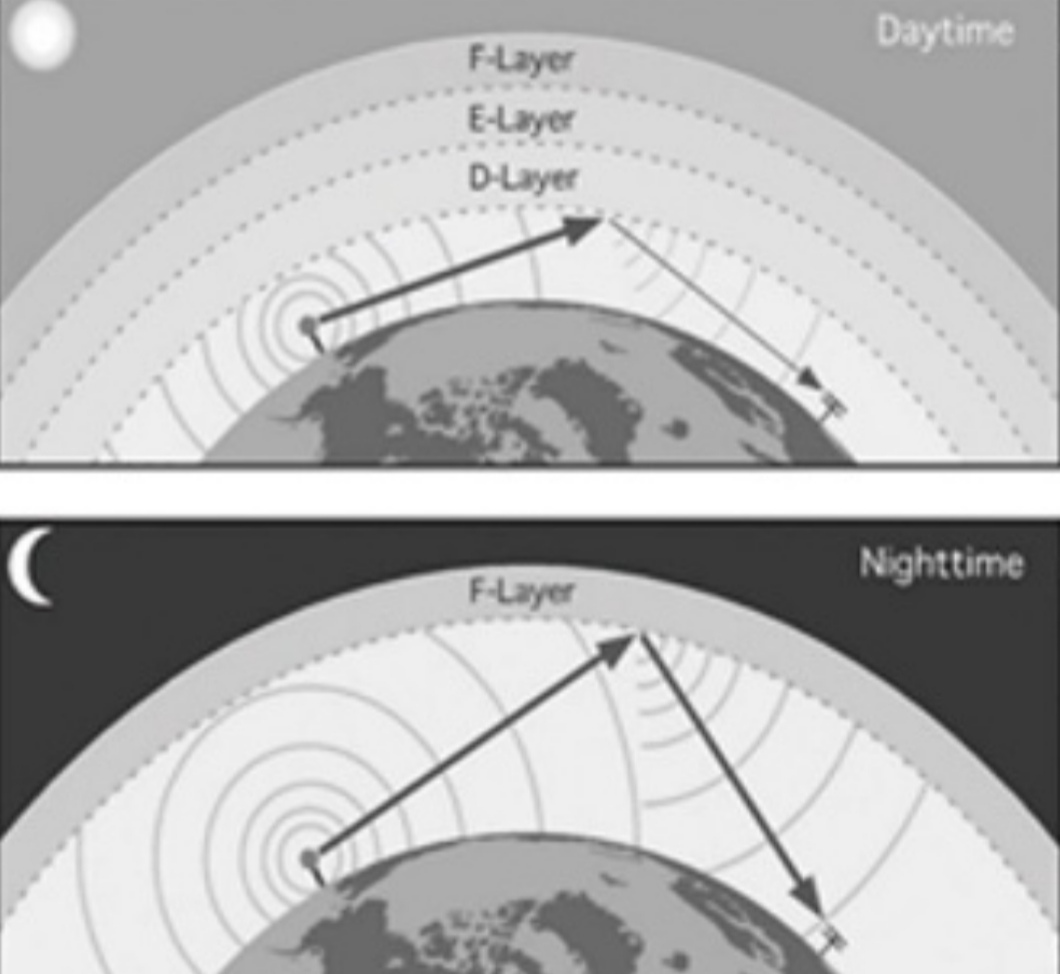
Ionosphere [80-400]km
an electrically charged layer, Overlaps with Thermosphere, site of Aurora
No influence on daily weather
Important for long wave radio transmission that Travel in straight lines & bounce off the Ionosphere
– at night F layer reflects AM waves
– in daylight D layer absorbs AM waves
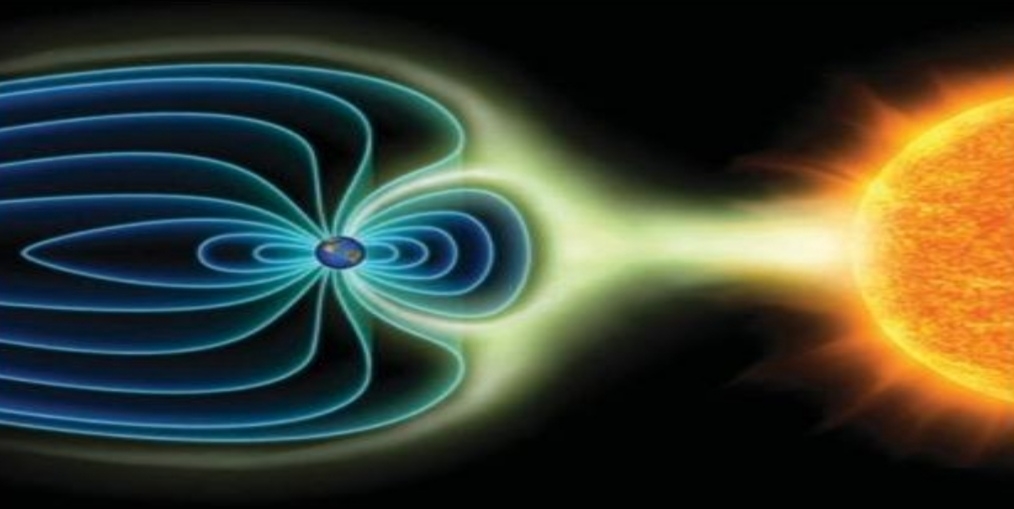
Aurora [100-400]Km
Closely correlated with solar-flares & Appear in the night sky as overlapping curtains
phenomenon that forms as energetic particles from sun & interact with Earth’s atm
Aurora boreal (northern lights)
Aurora austral (southern lights)
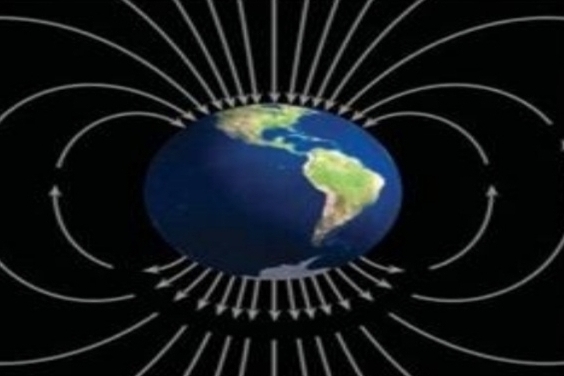
Geographic location is important (Earth’s magnetic poles)
produce by solar wind distorts Earth’s magnetic field into magnetosphere & These particles interact with gases to produce aurora
– solar wind stream of charged particles
– magnetosphere has teardrop shape

The End