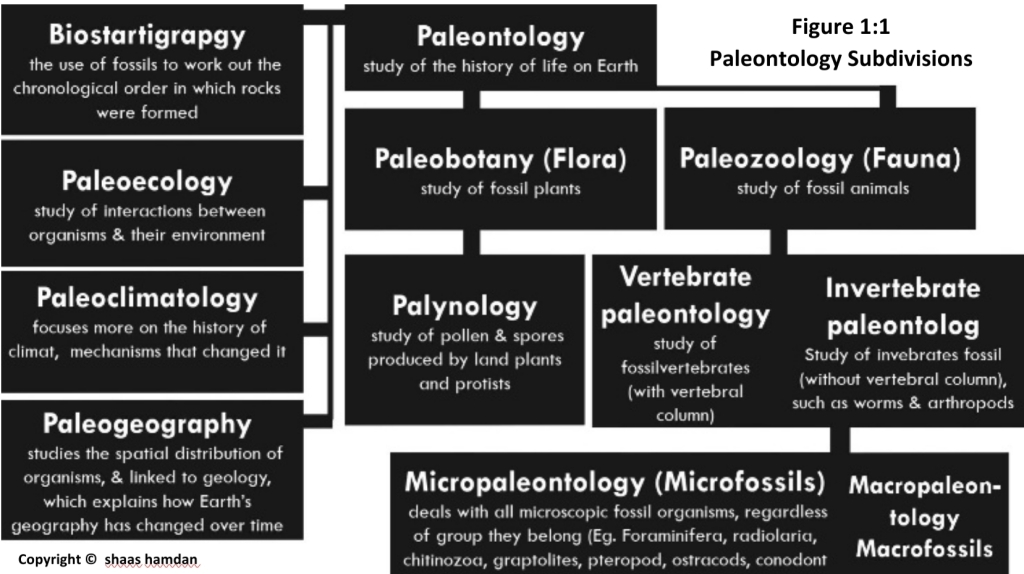
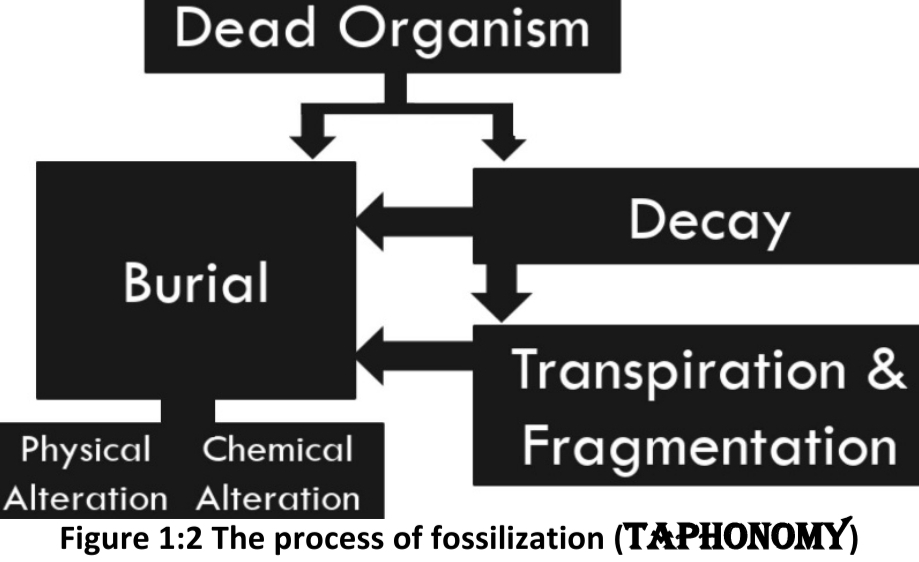
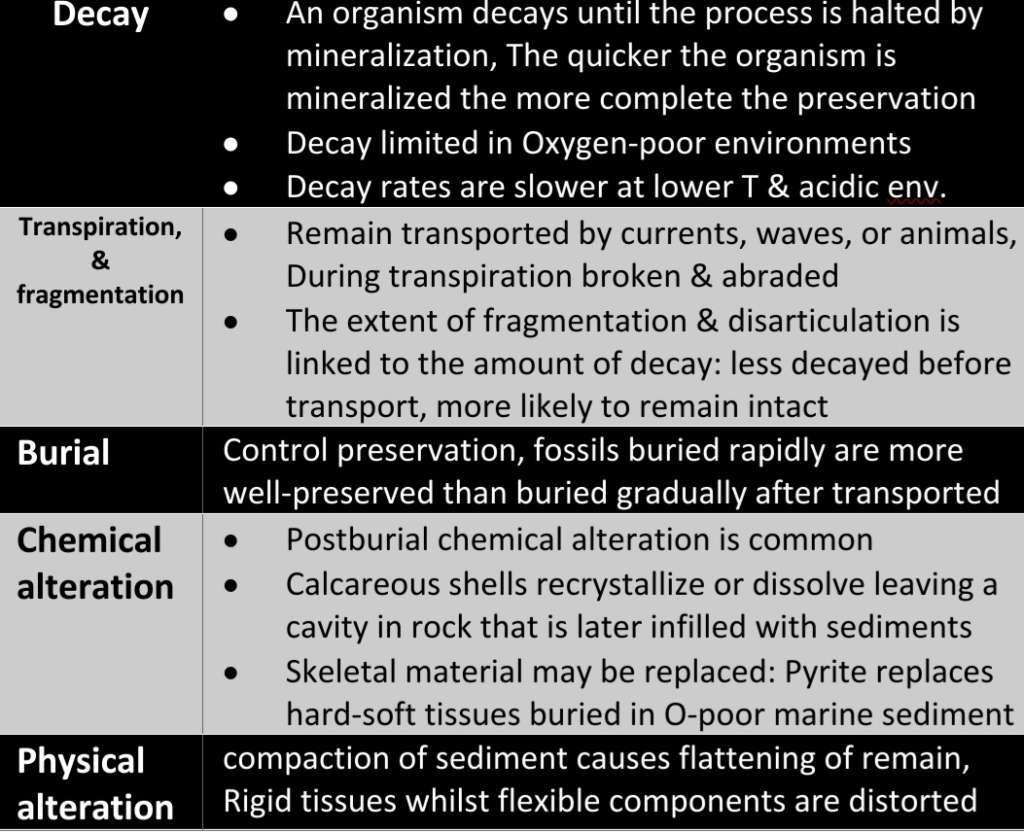
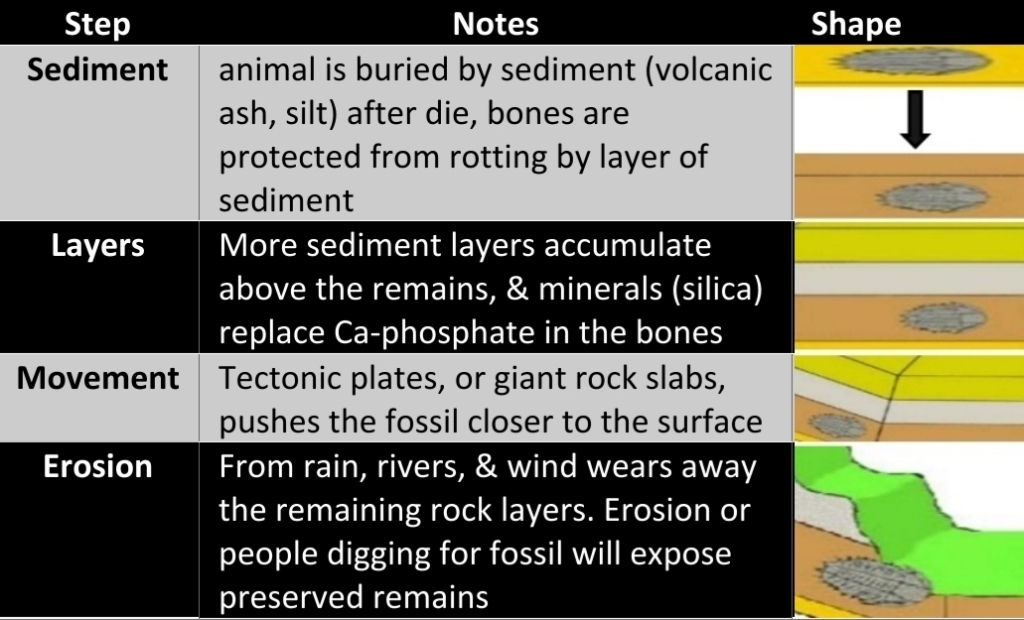
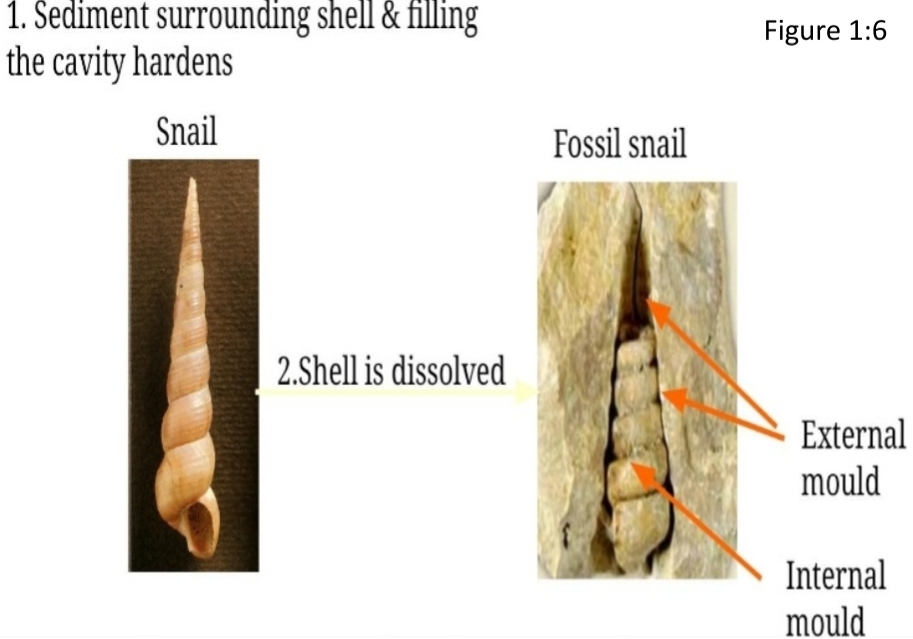
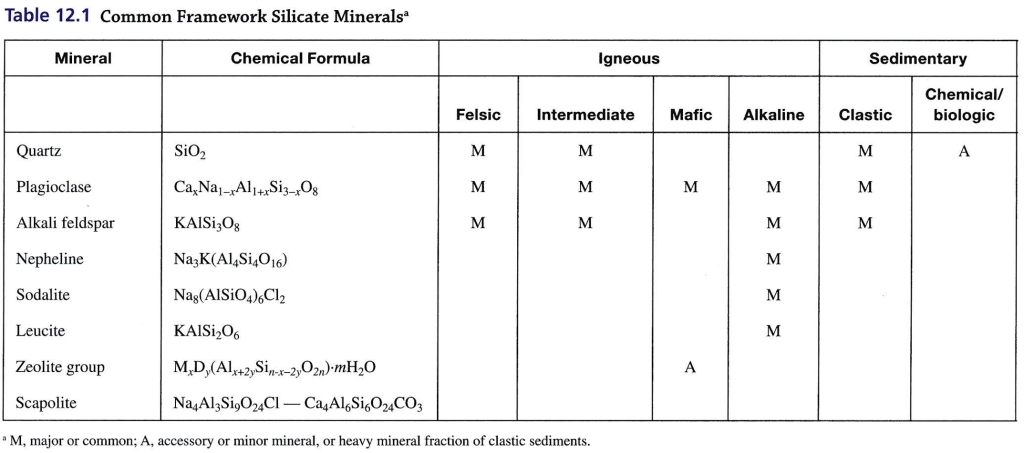
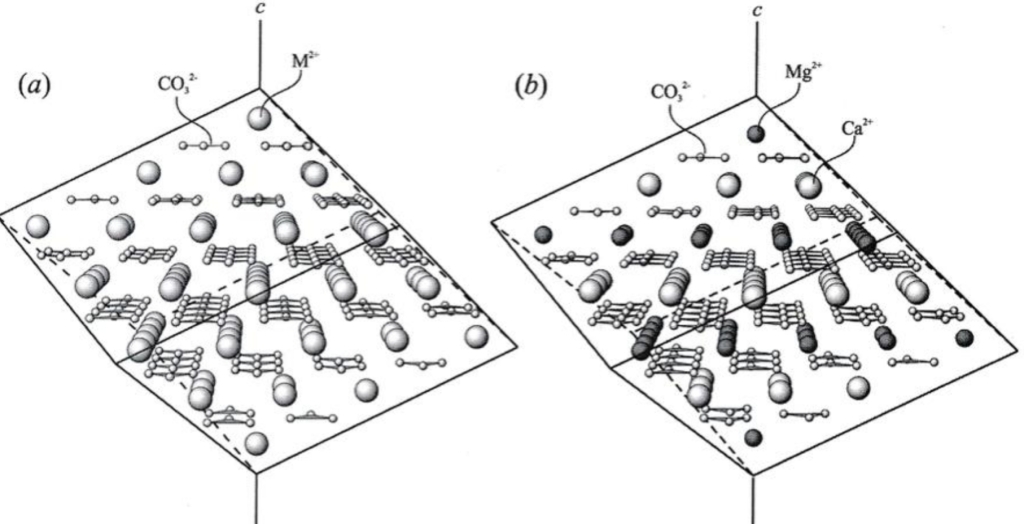
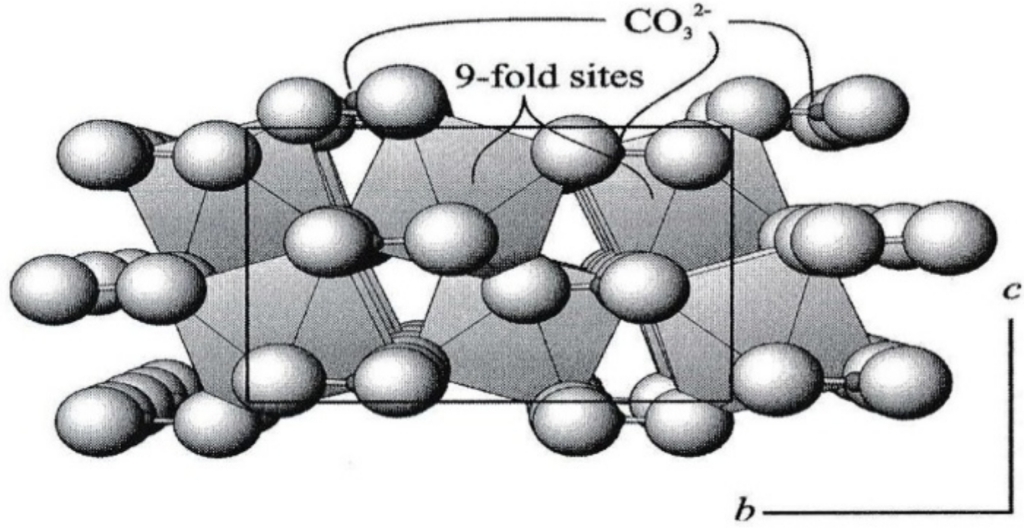
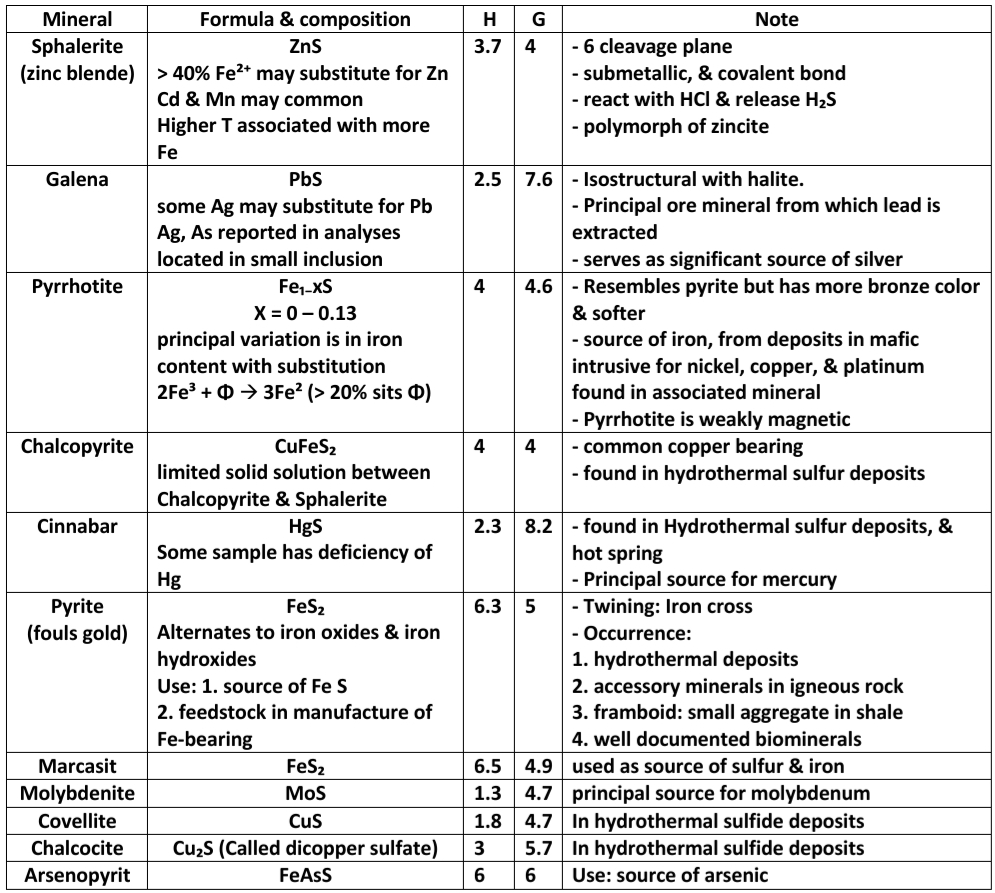
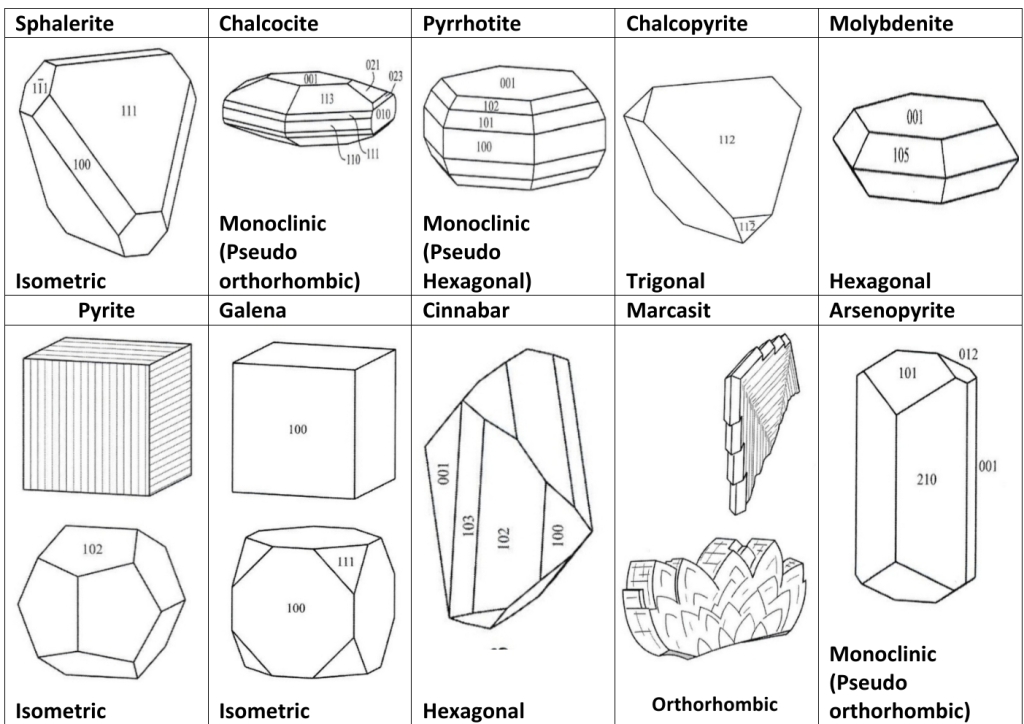
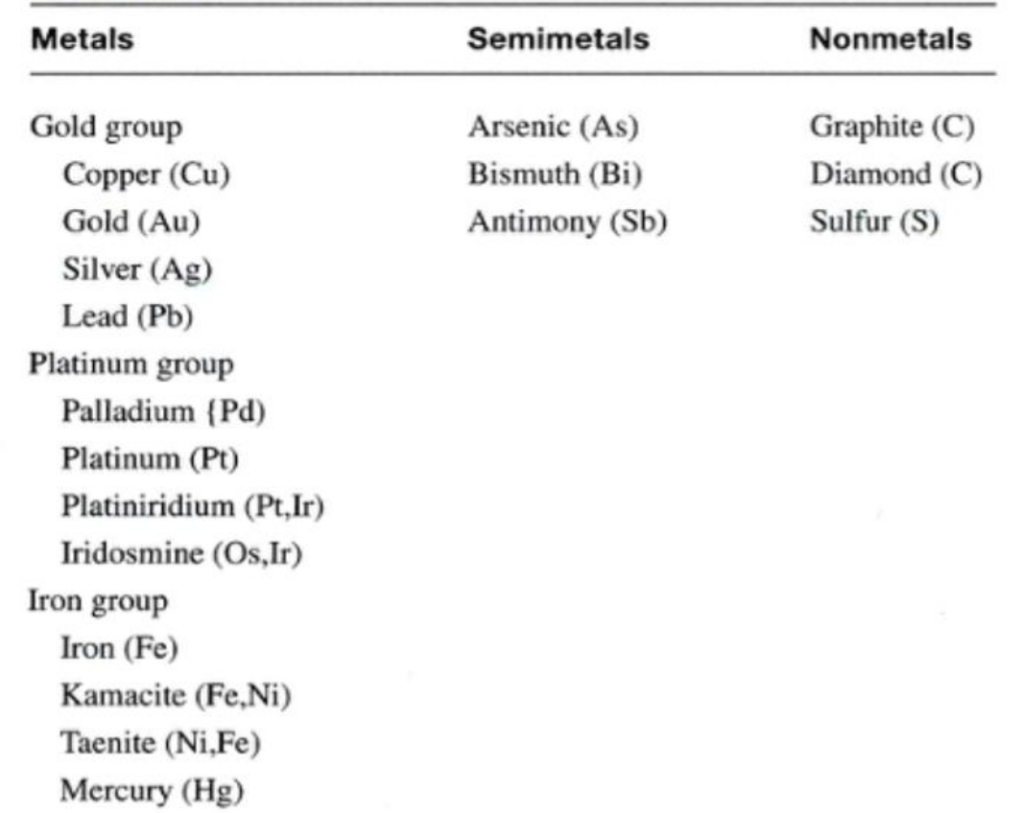
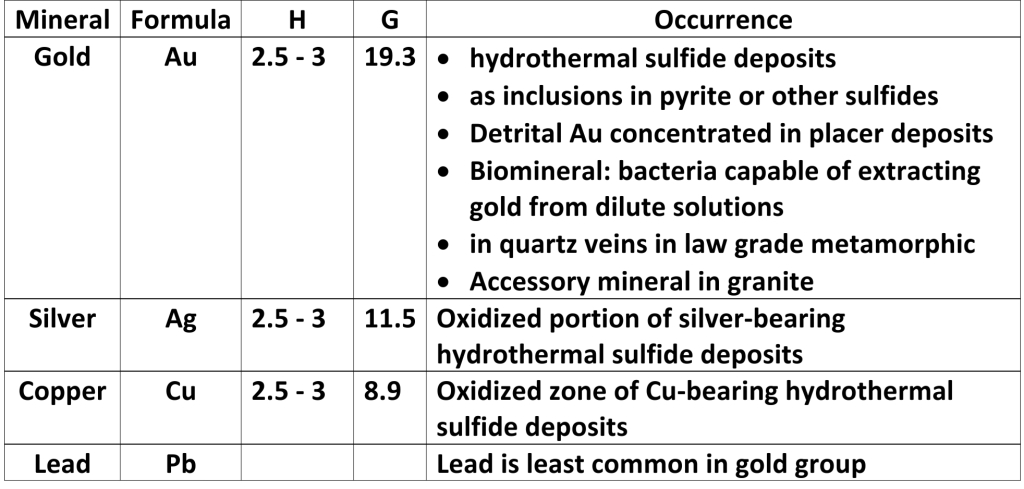
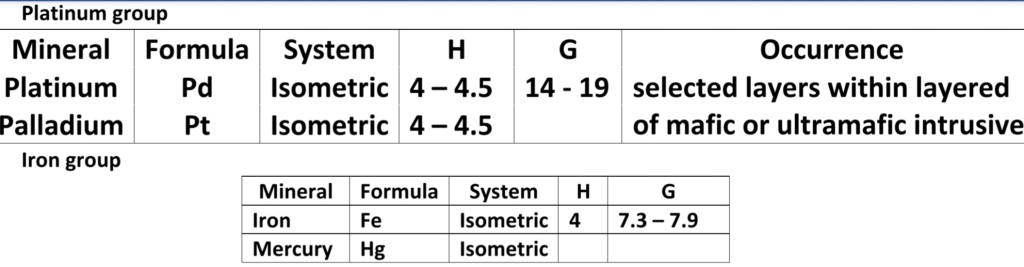
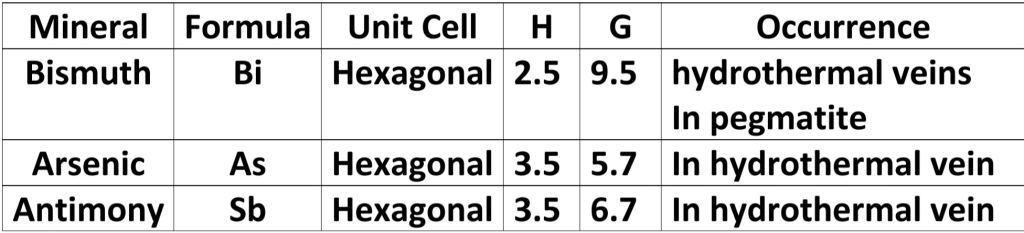
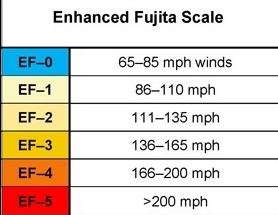
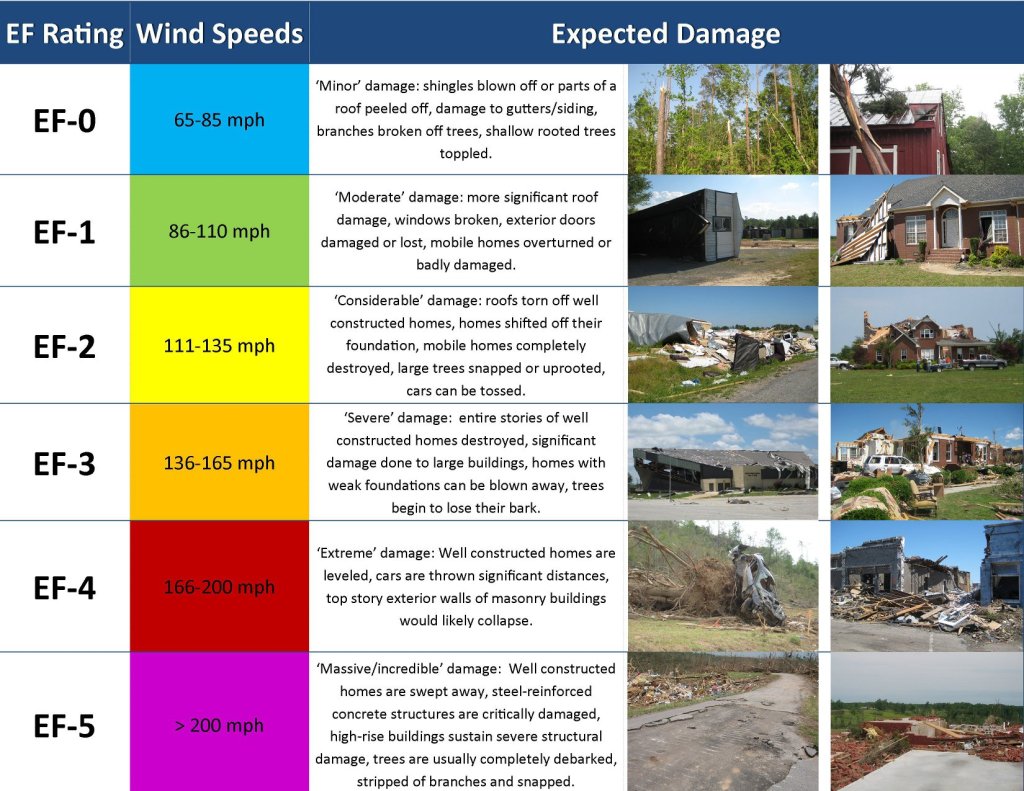
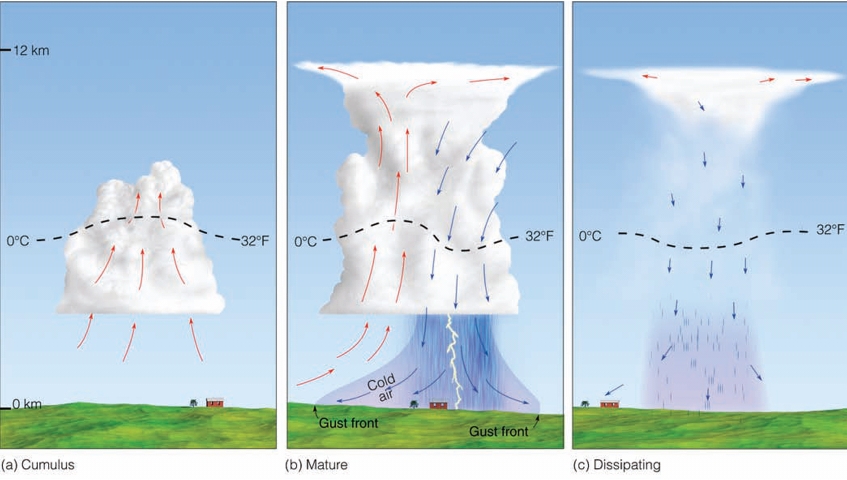
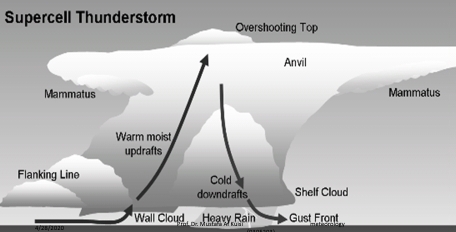
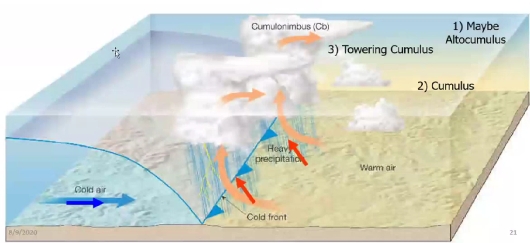
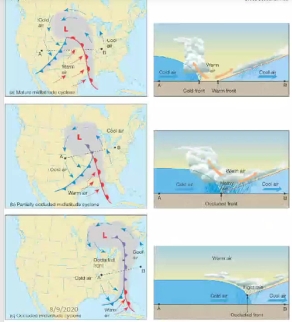
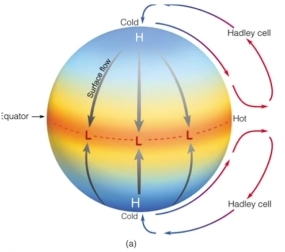
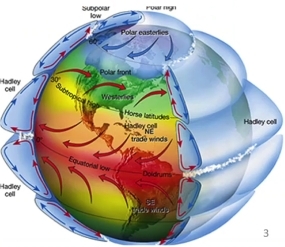
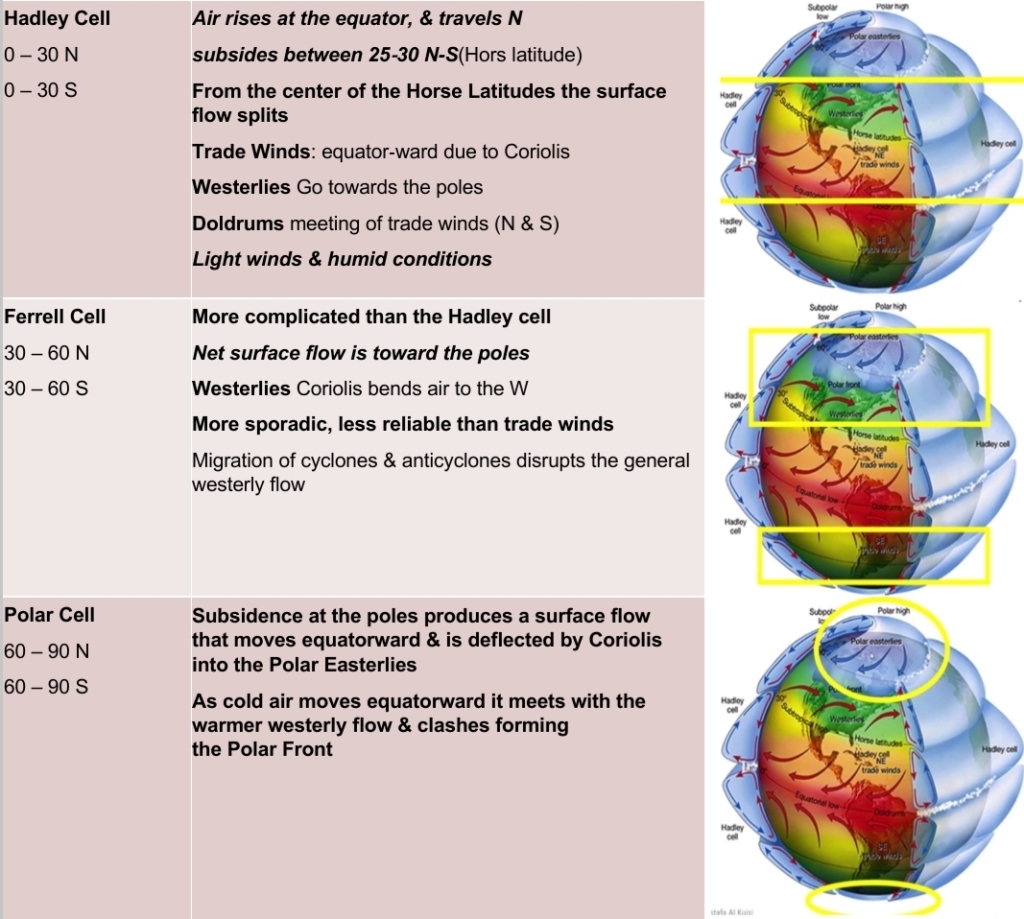
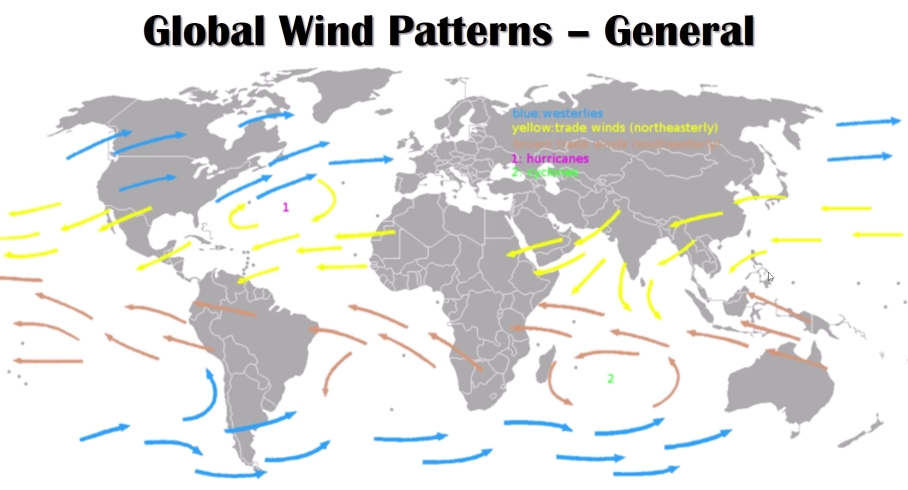
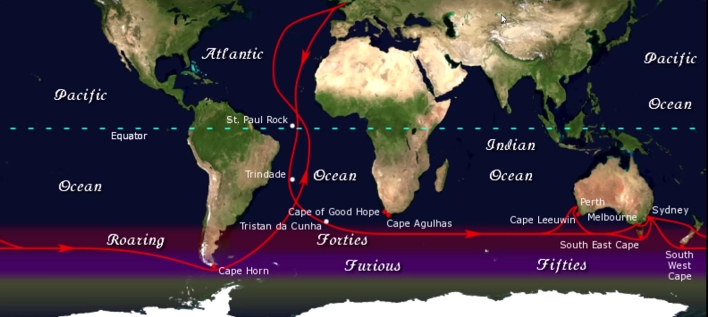
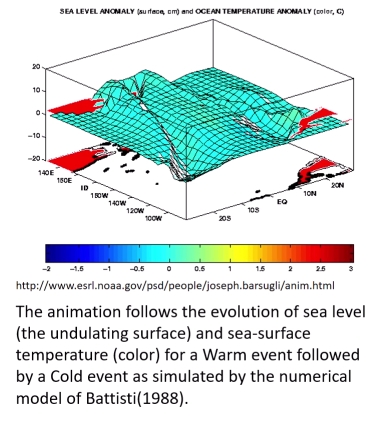
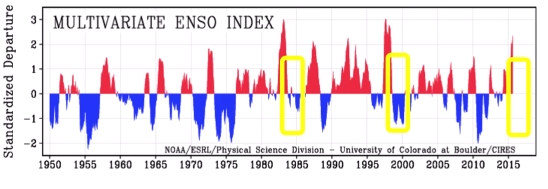
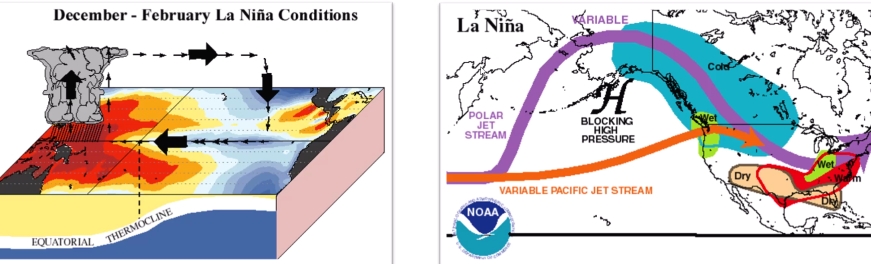
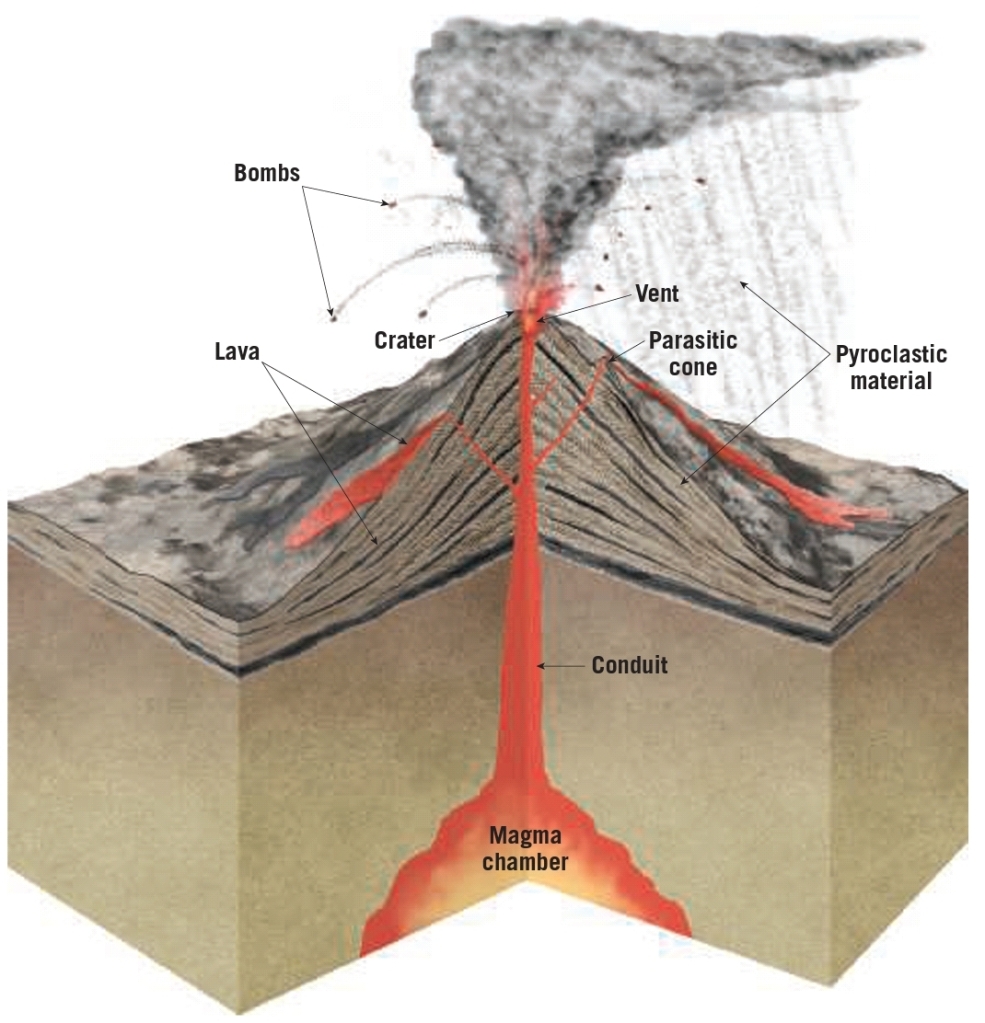
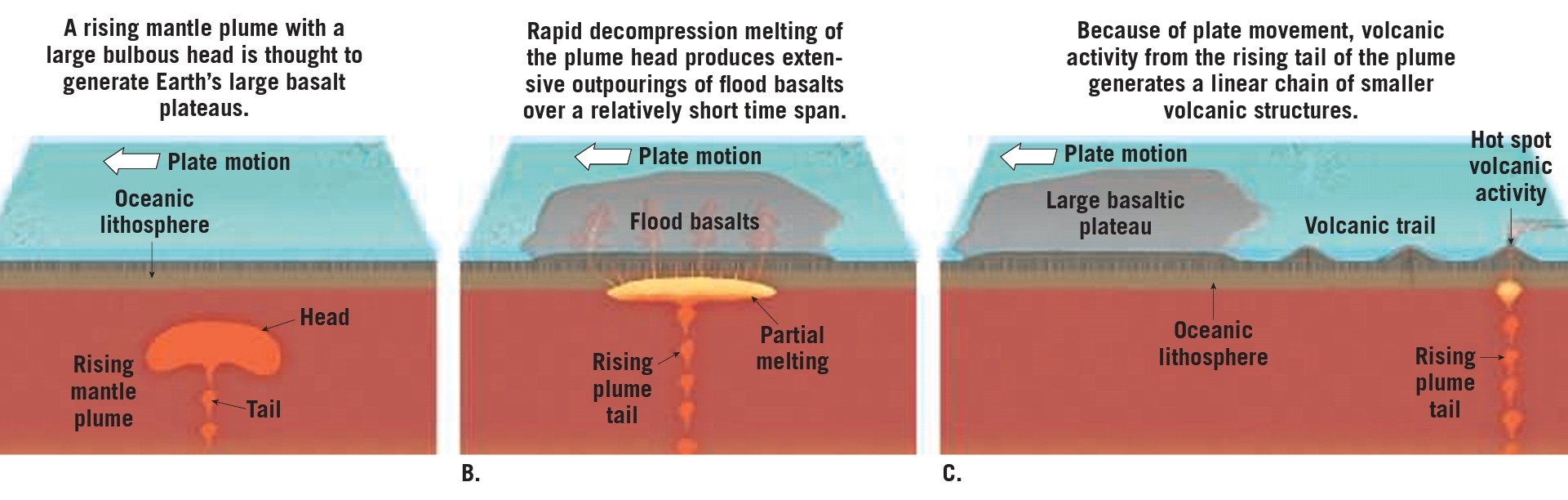
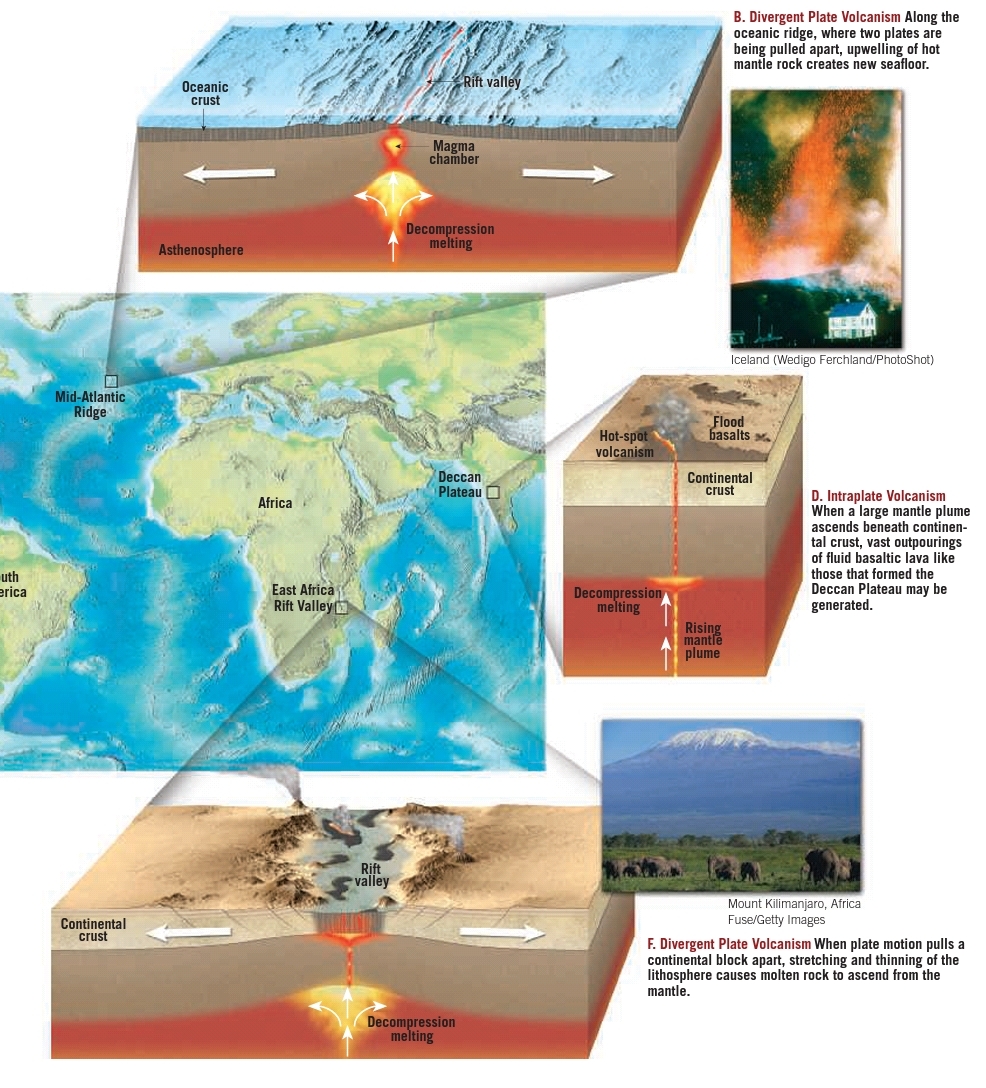
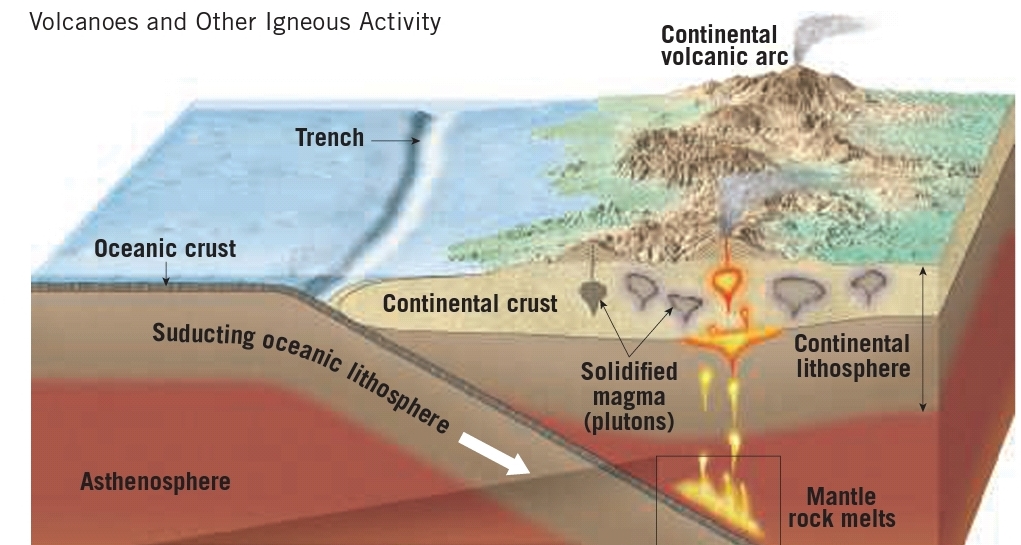
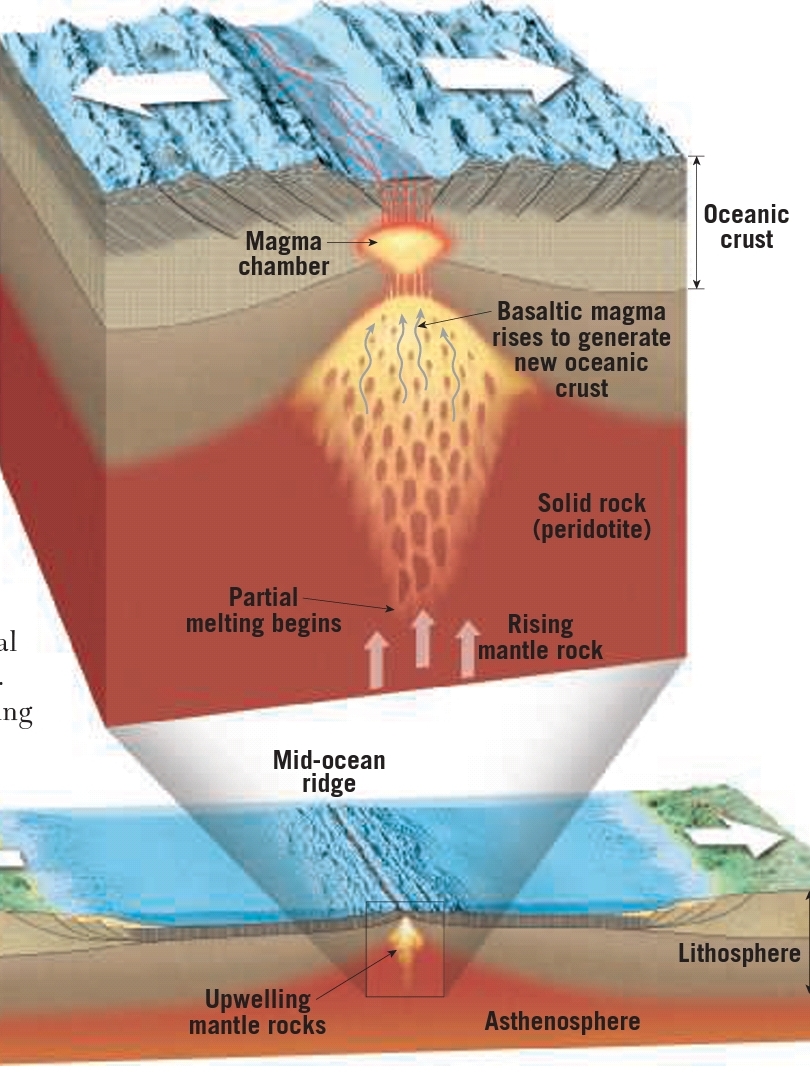
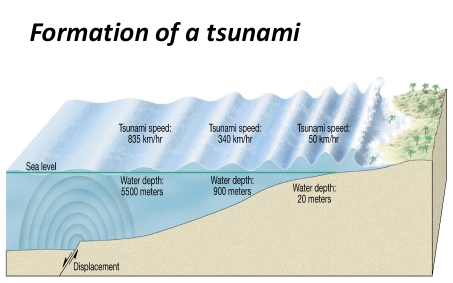
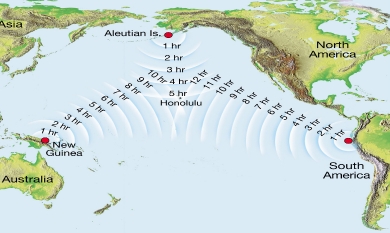
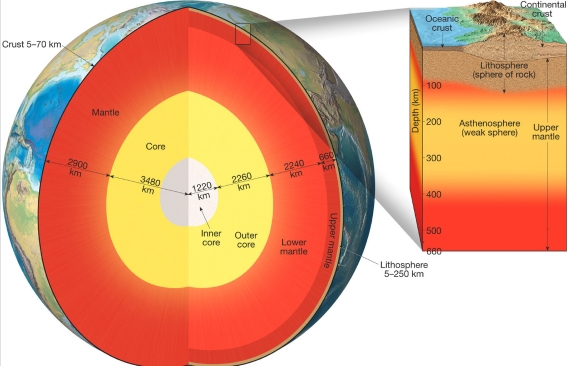
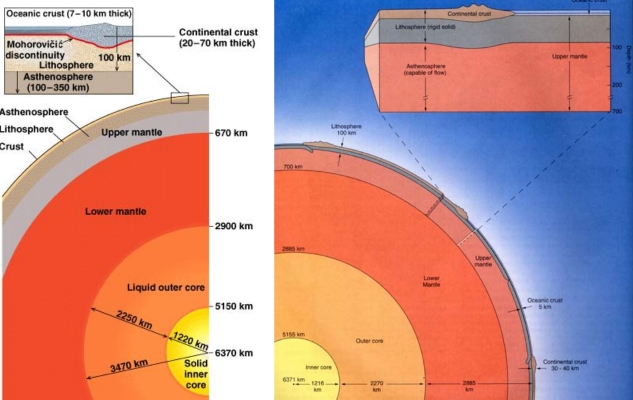
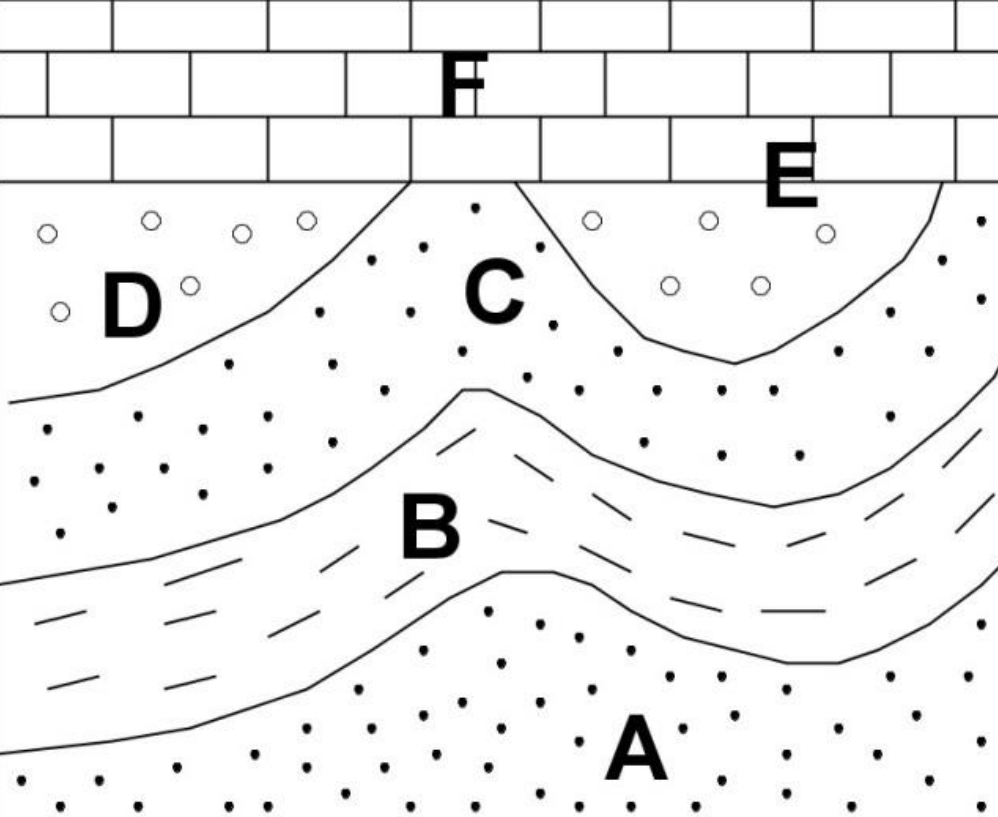
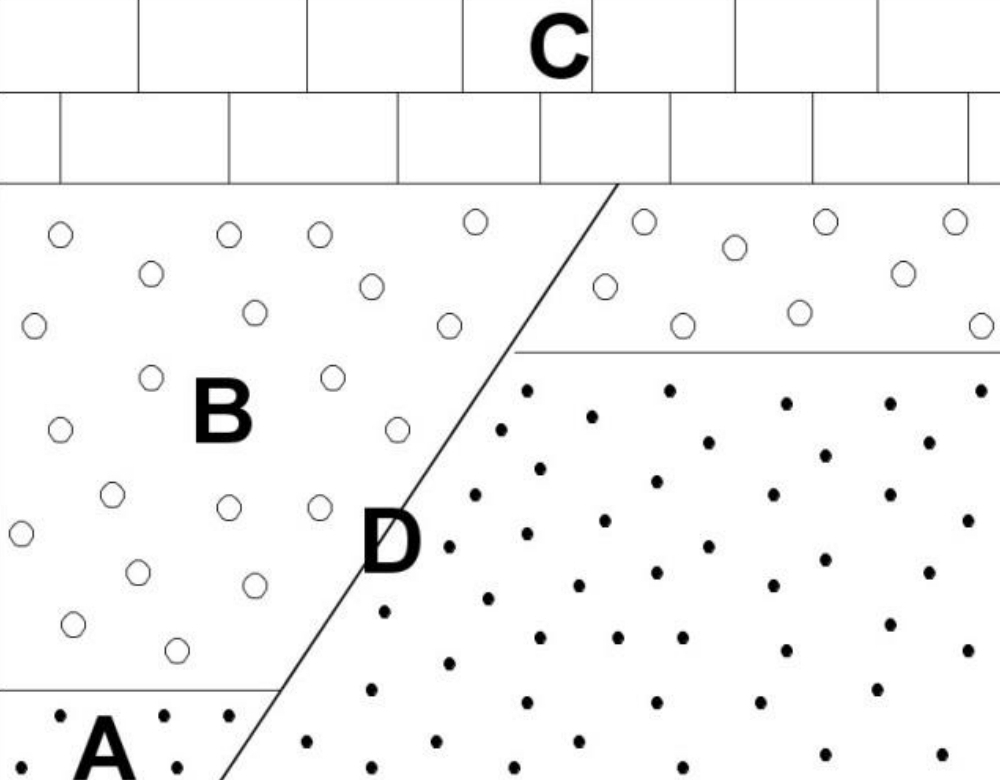
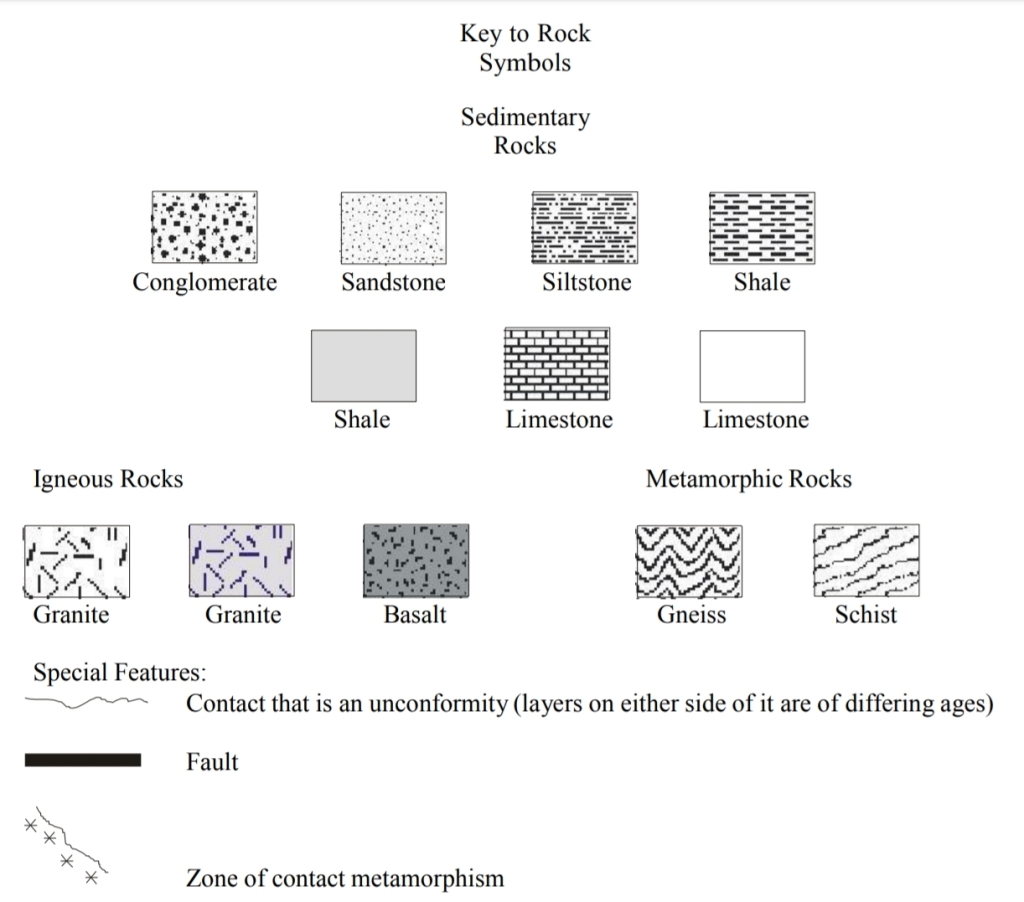
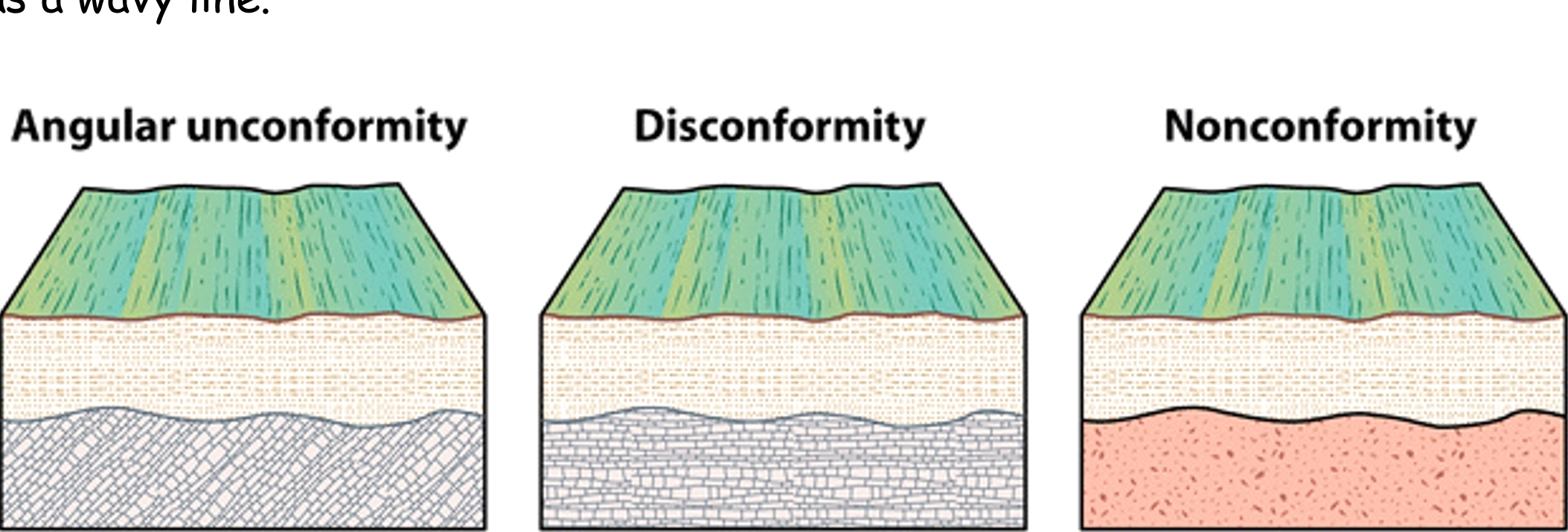
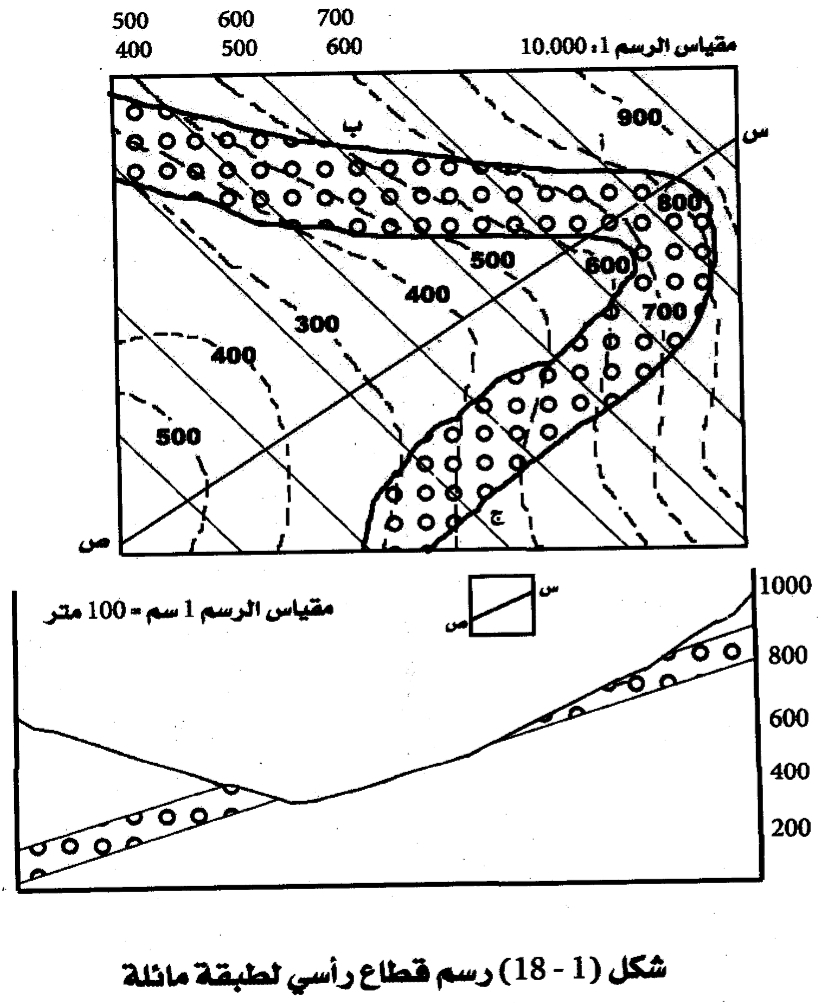
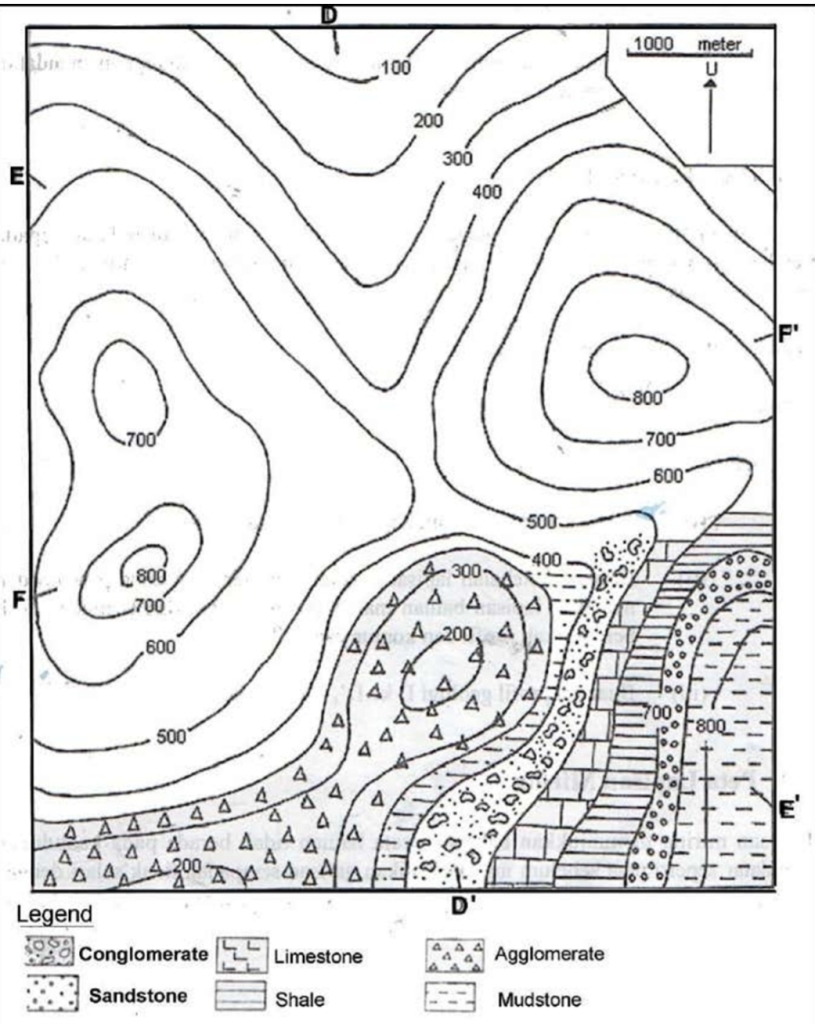
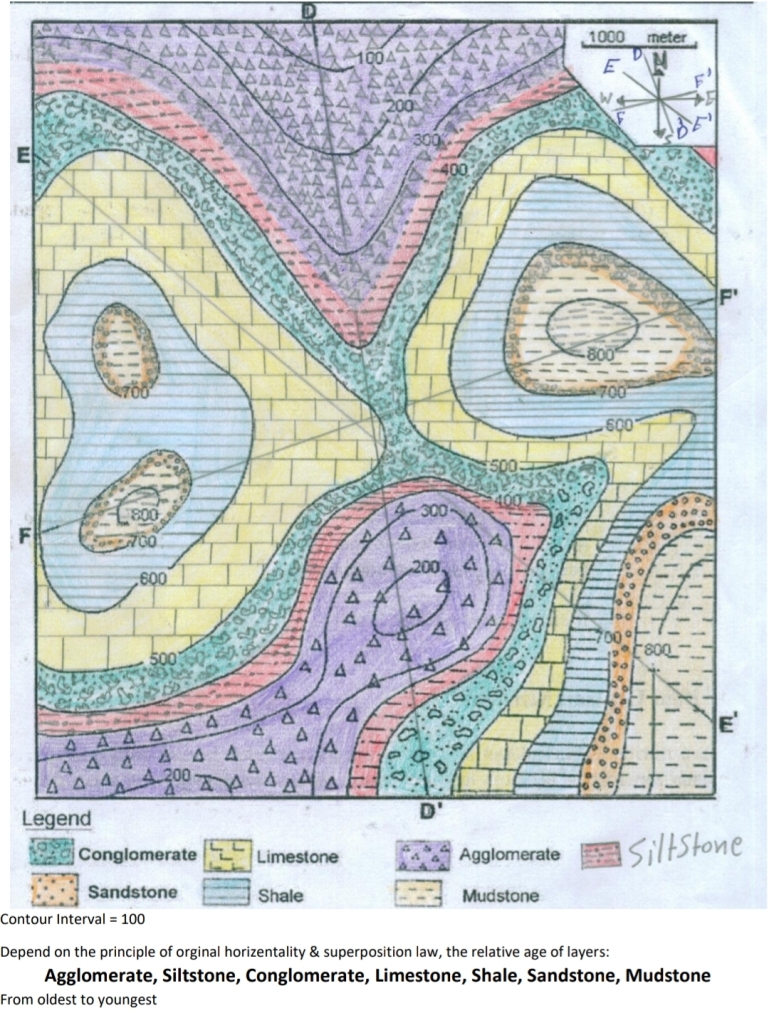
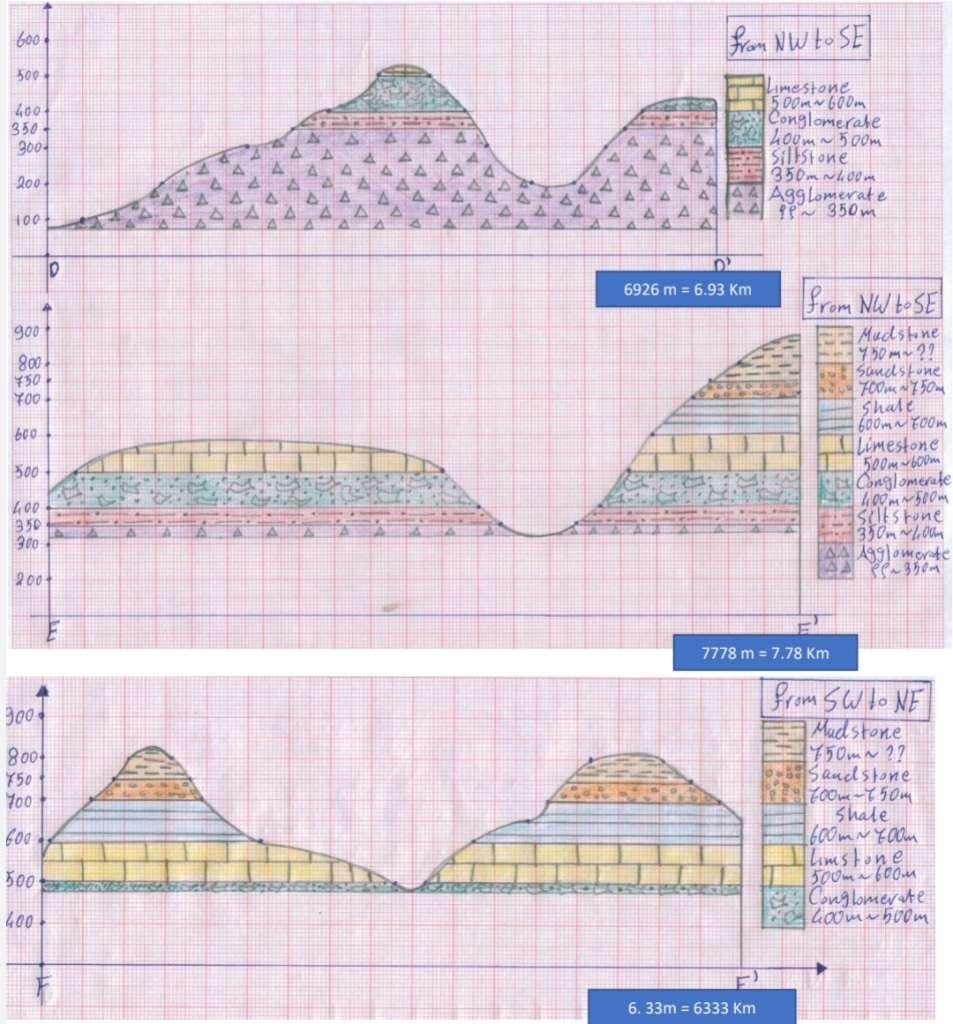
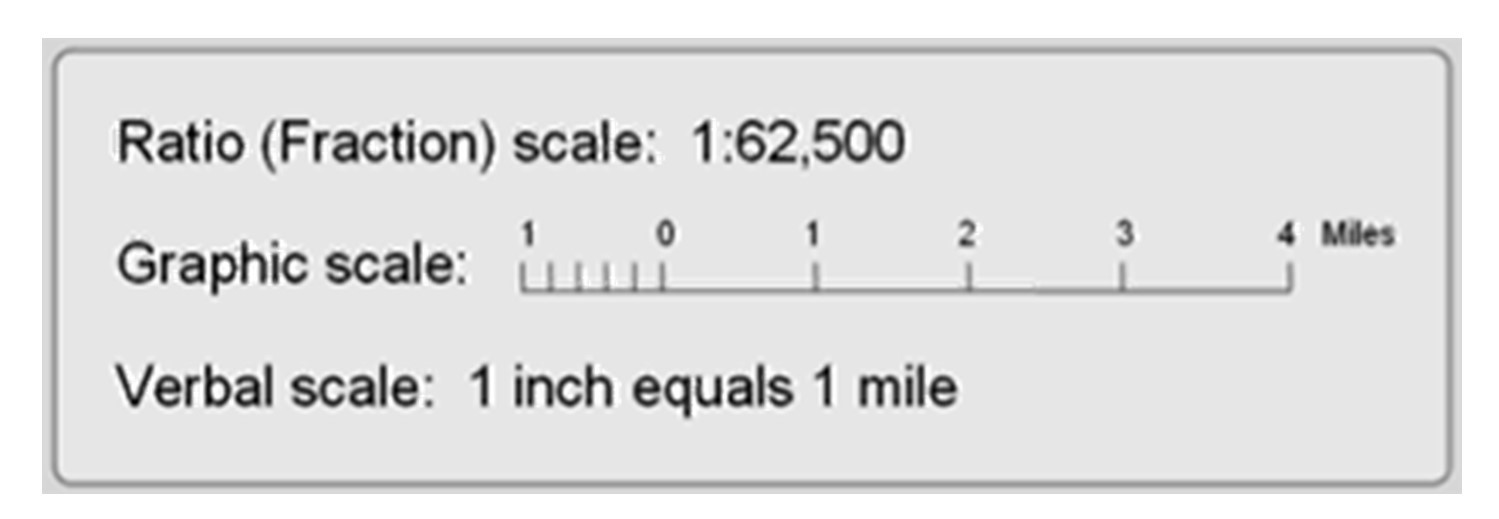
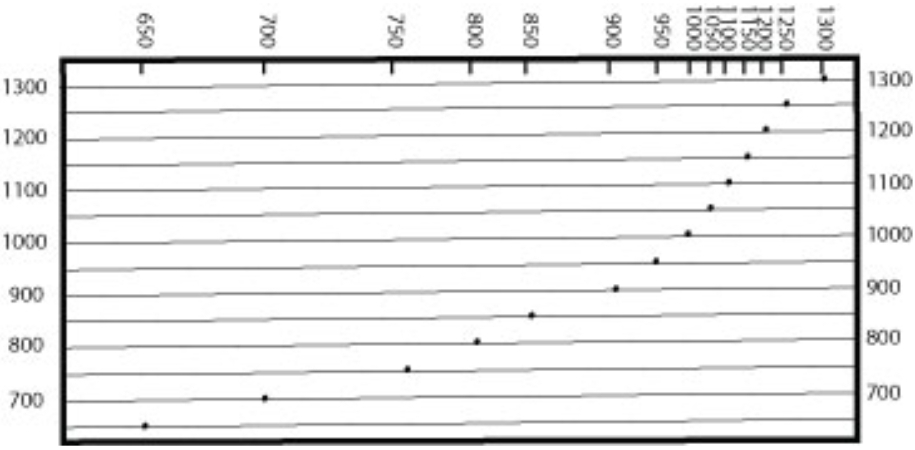
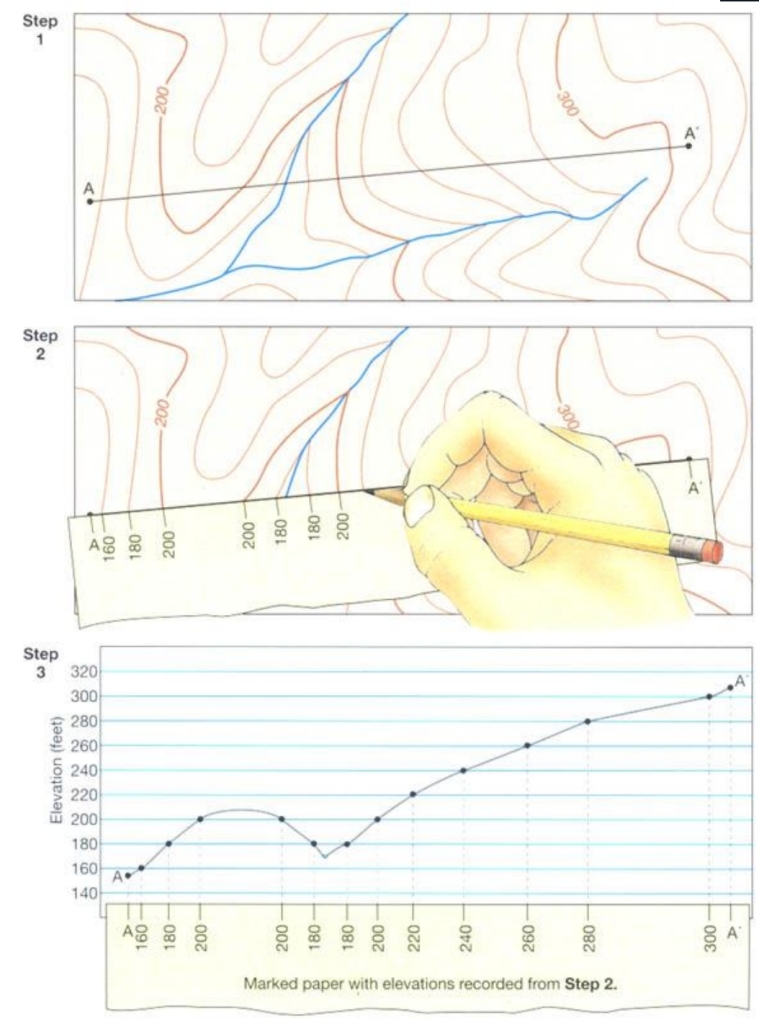
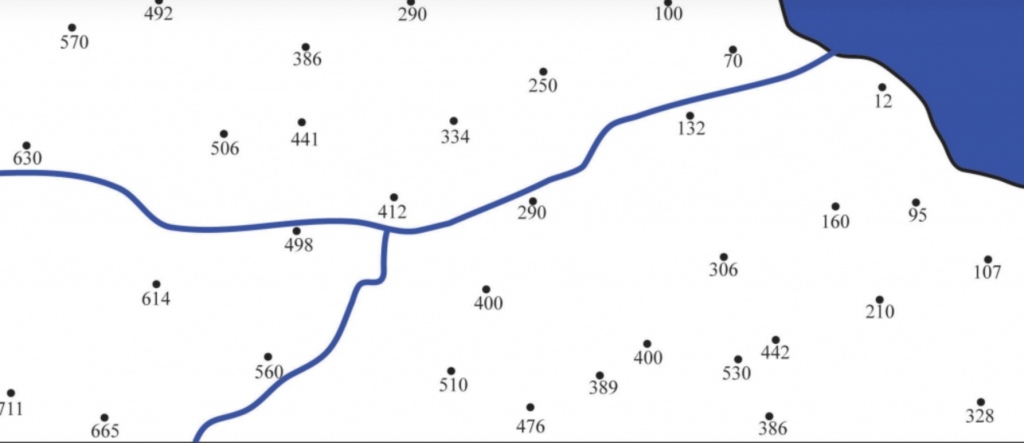
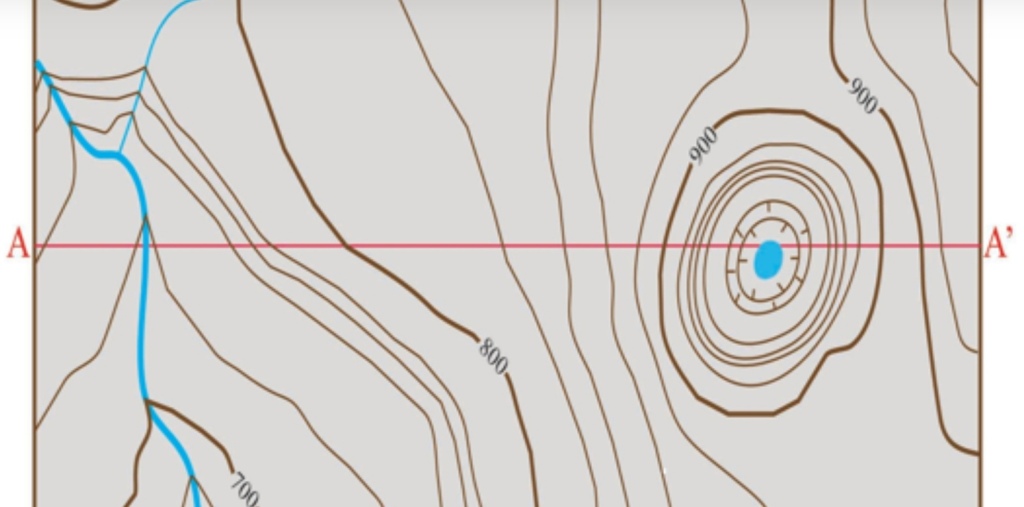
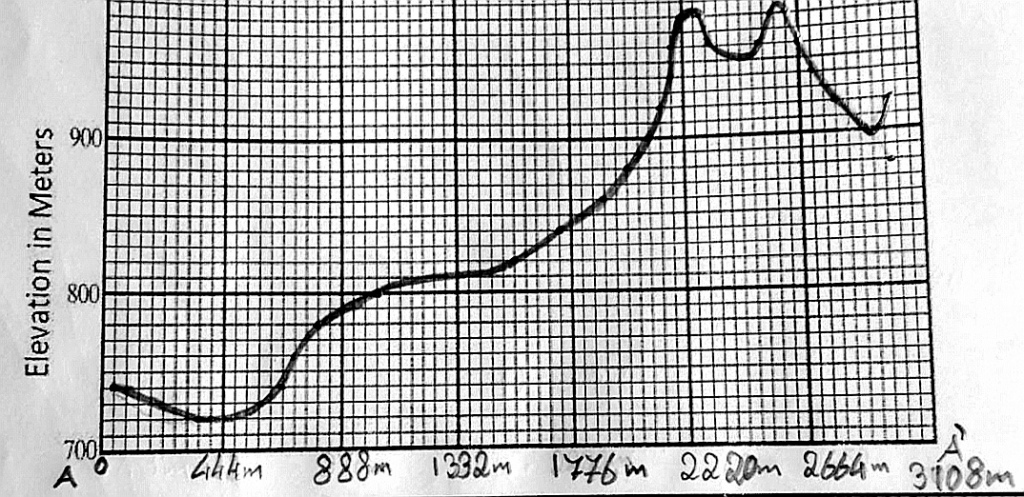
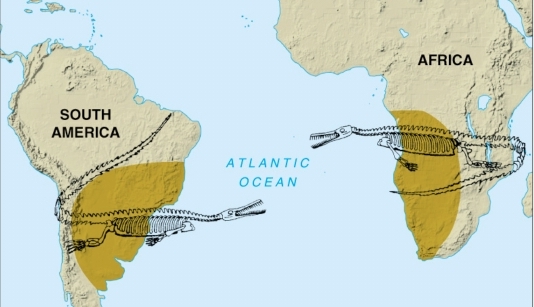
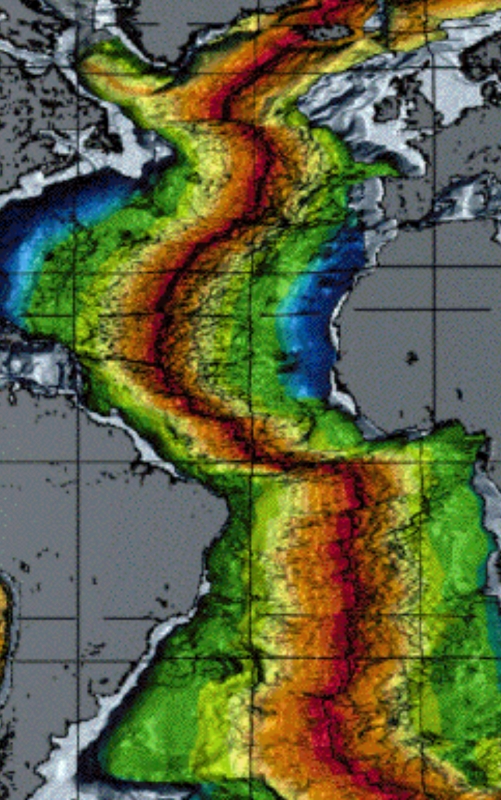
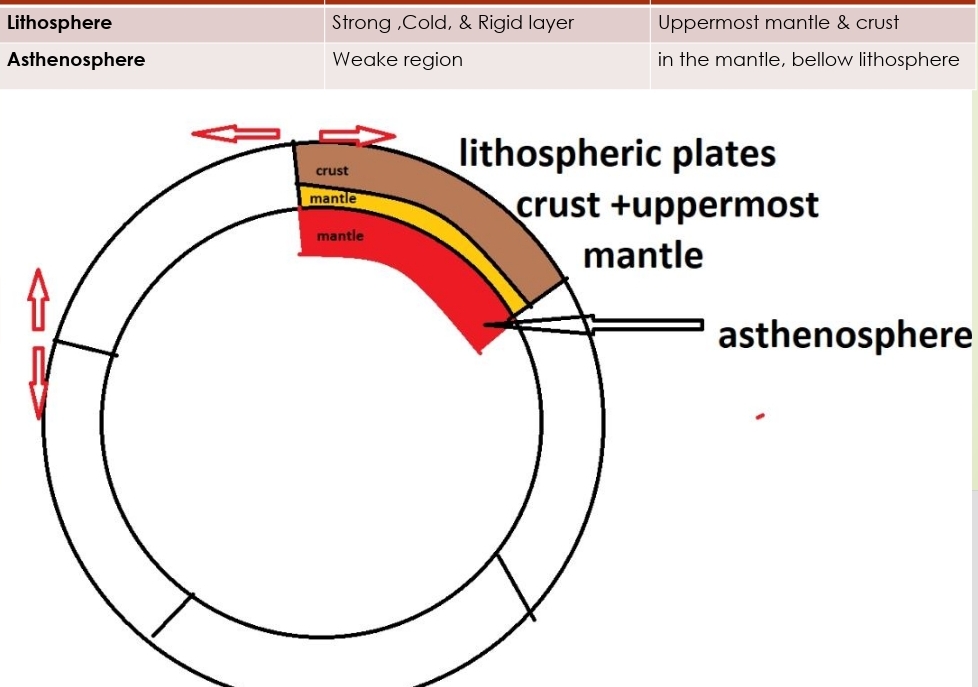
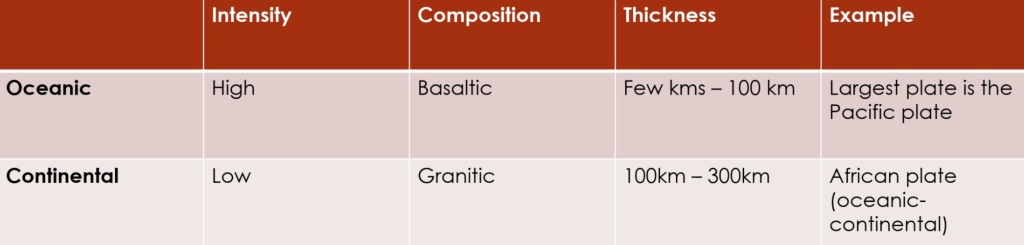
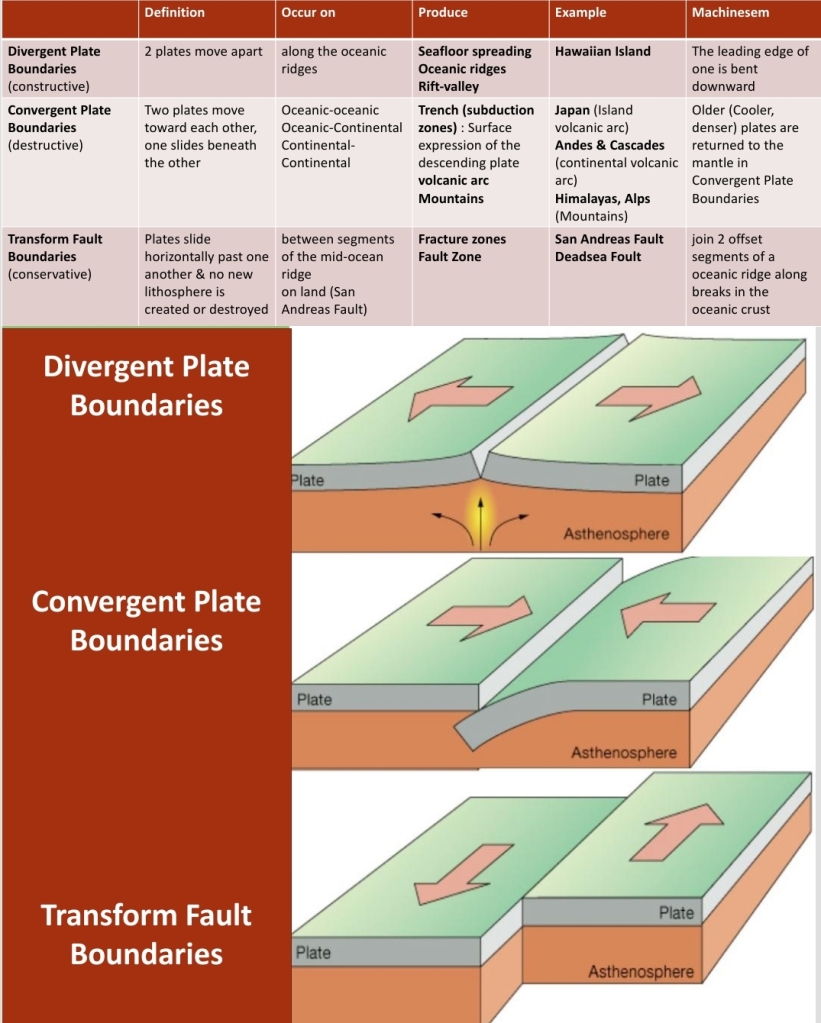
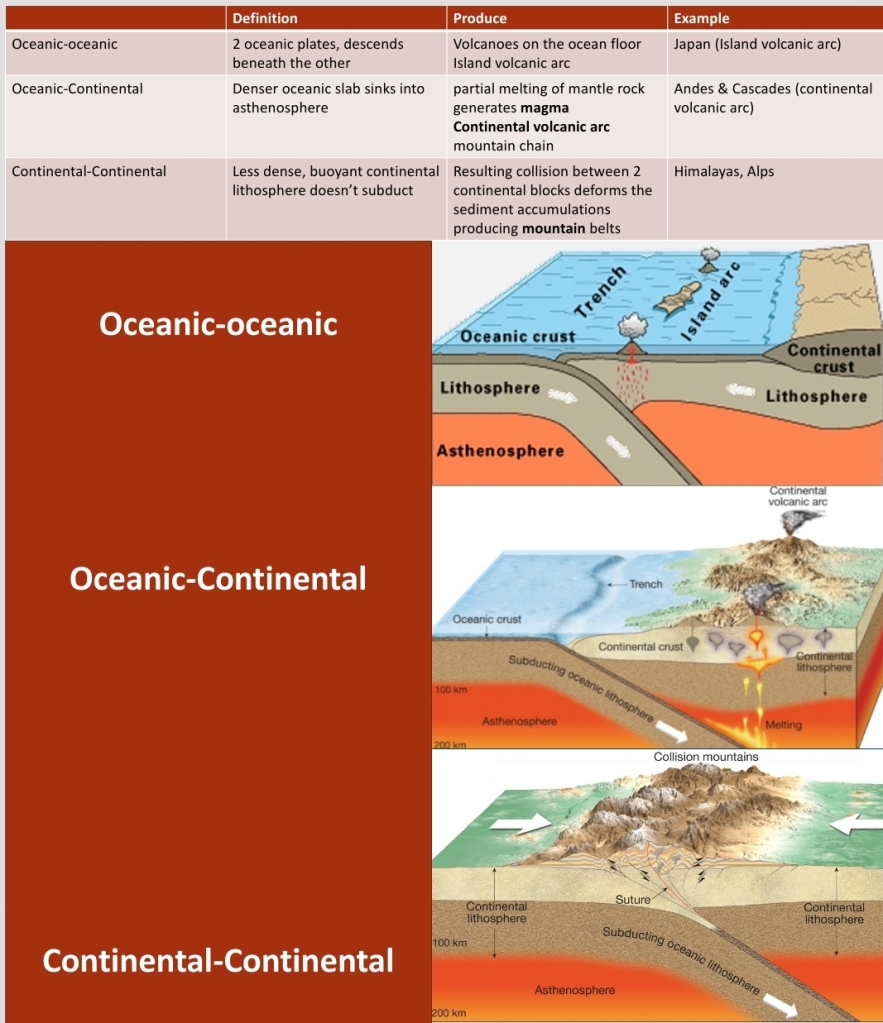
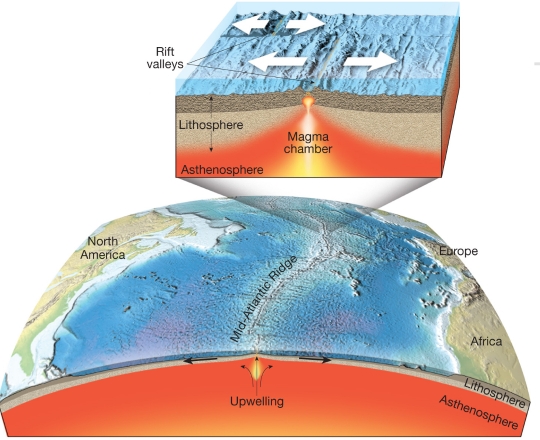
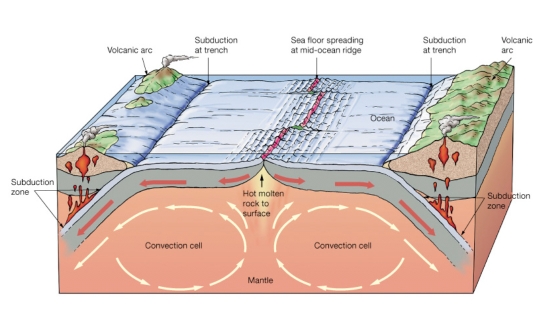
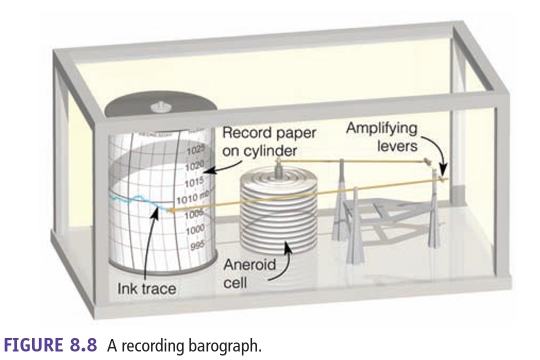
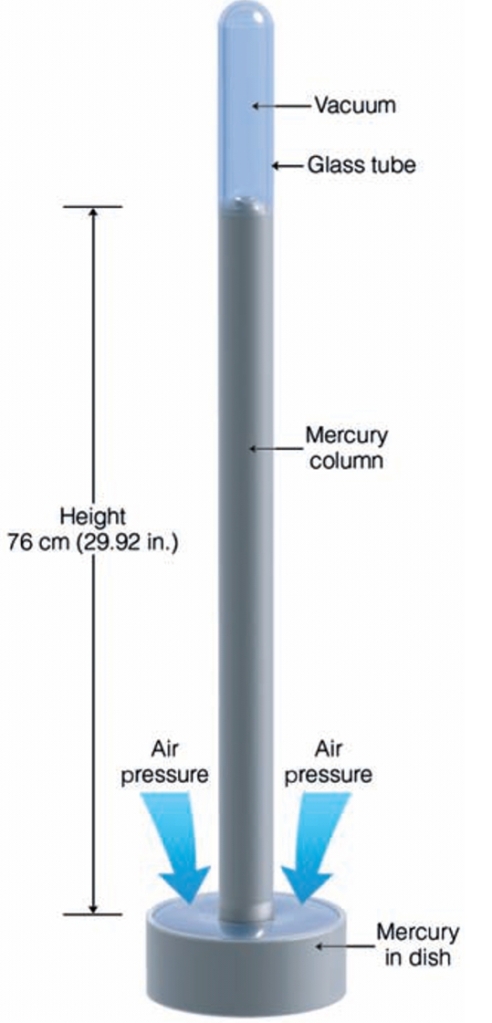
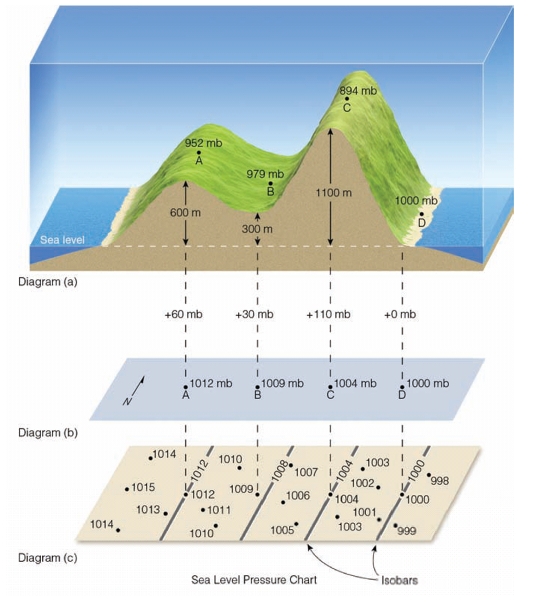
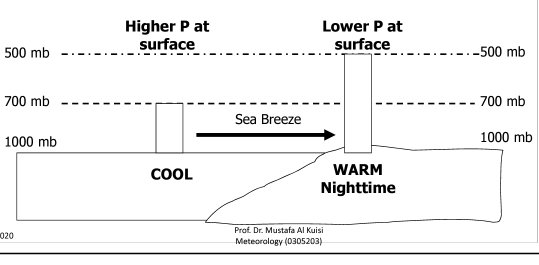
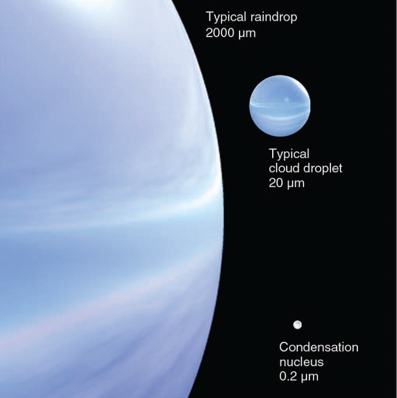
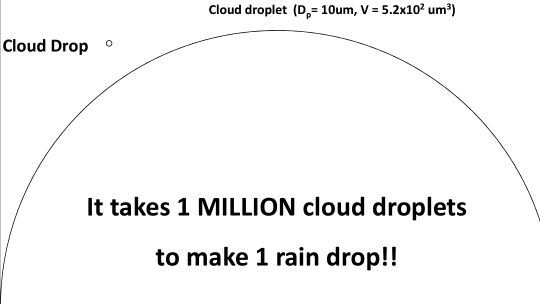
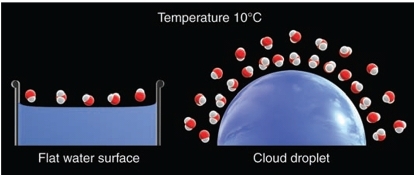
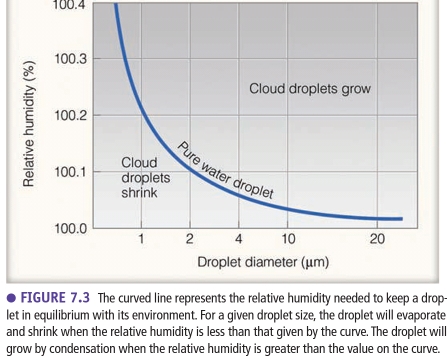
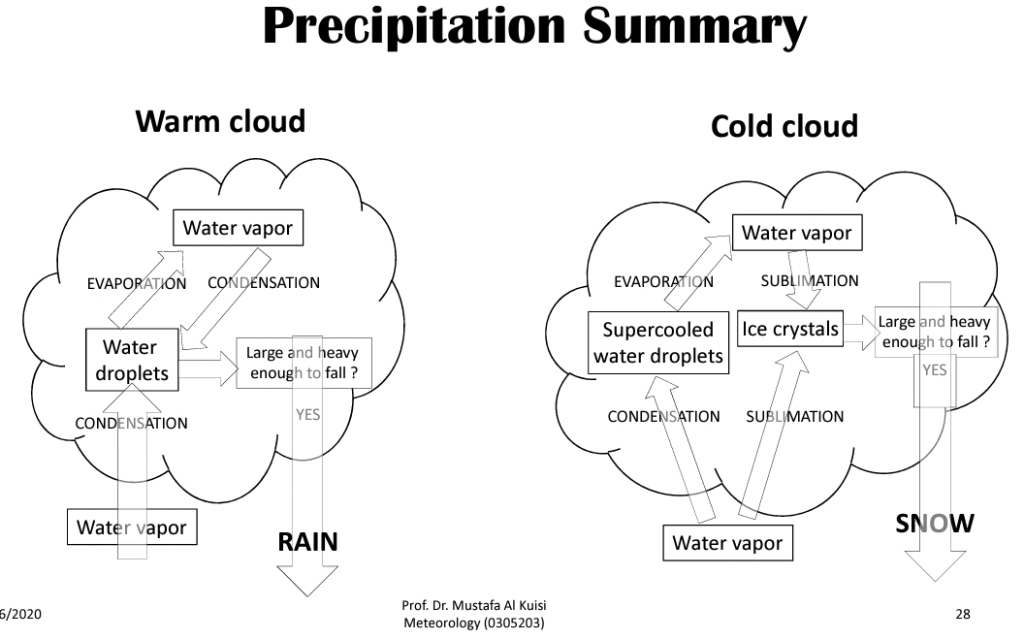
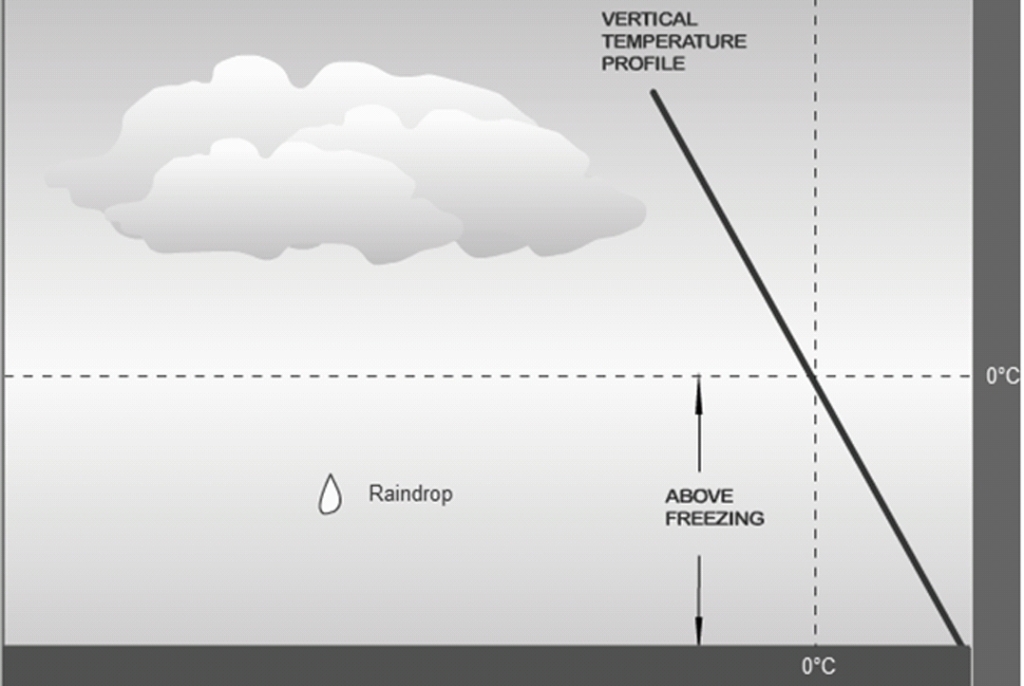
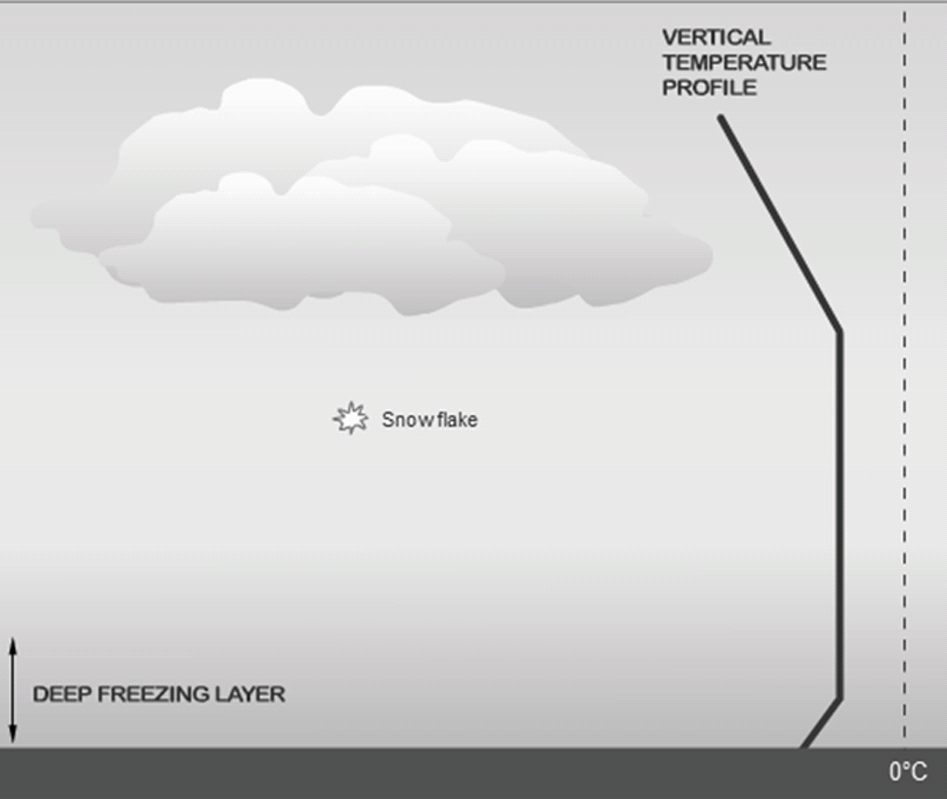
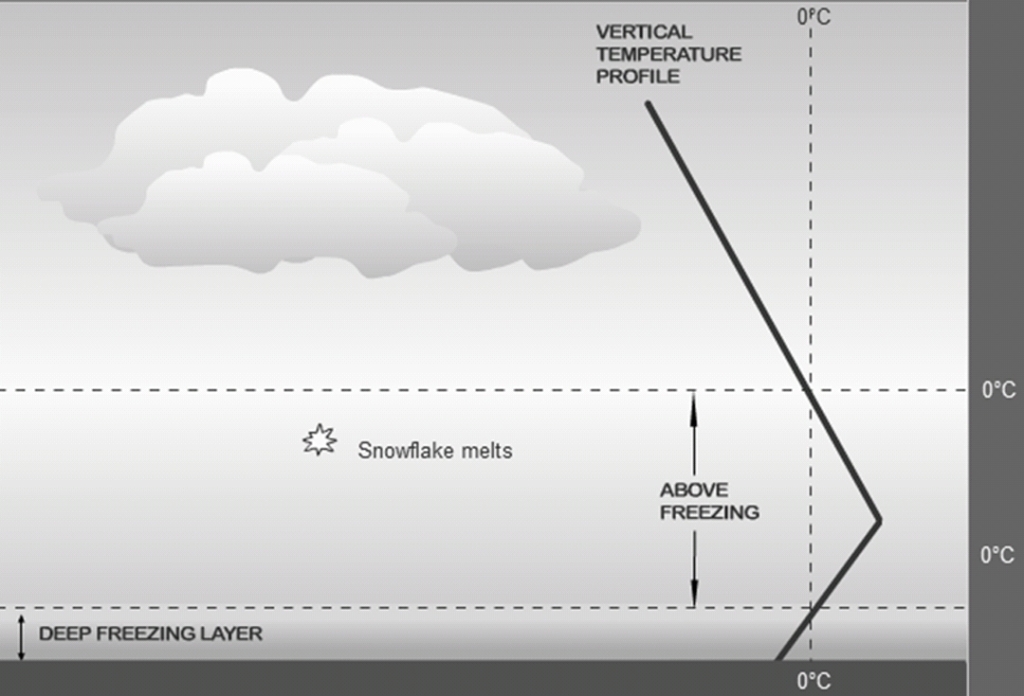
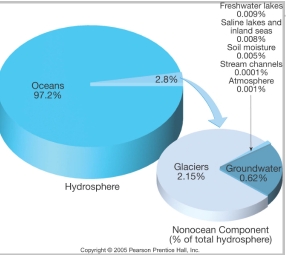
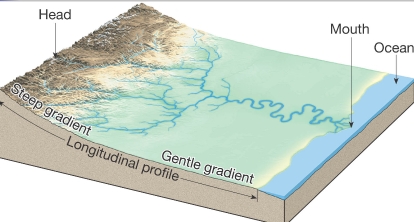
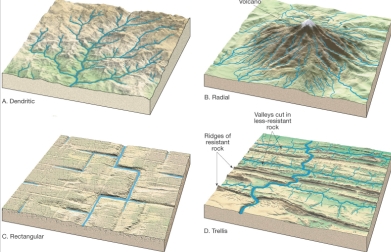
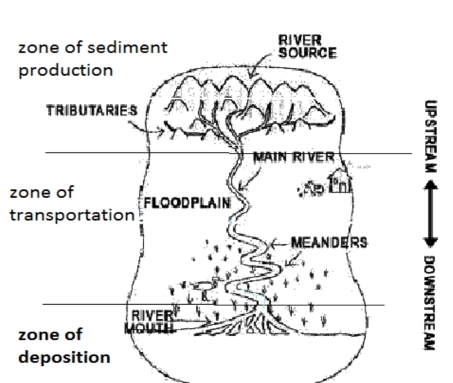
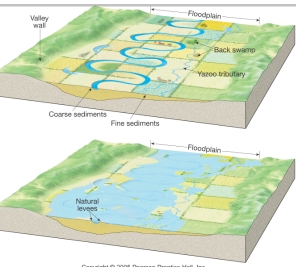
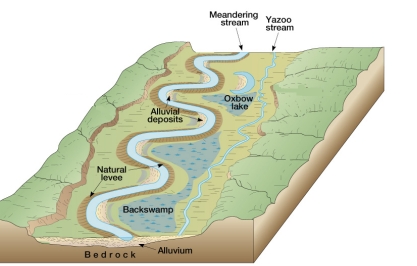
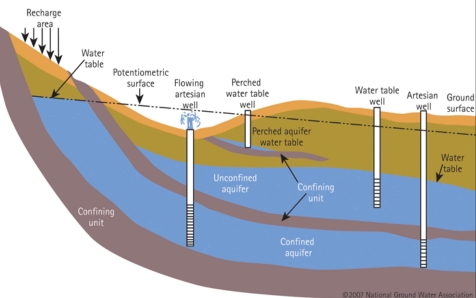
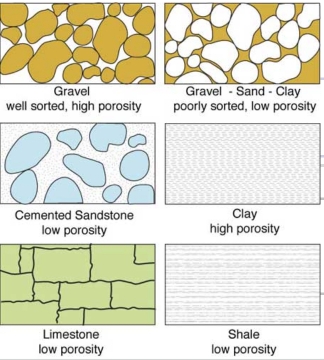
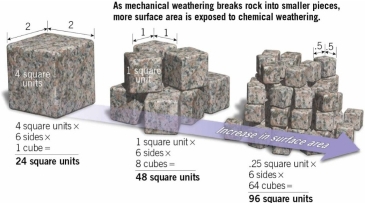
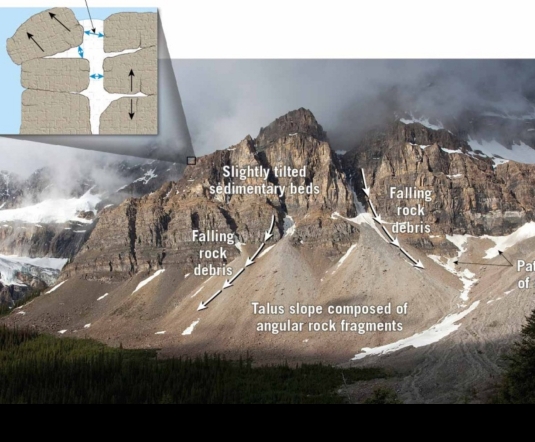
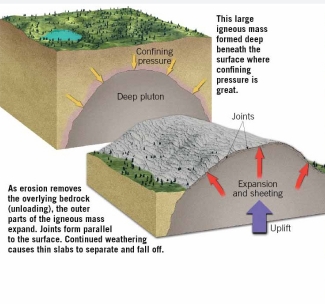
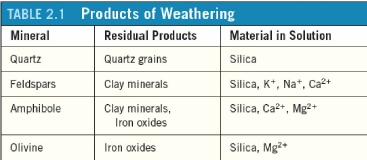
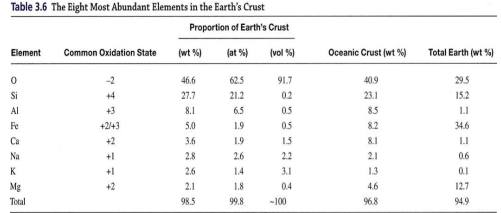
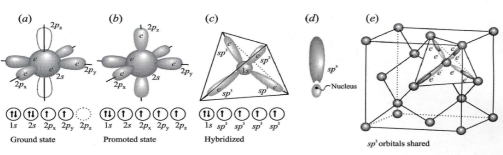
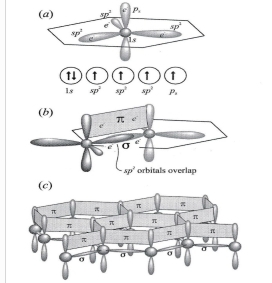
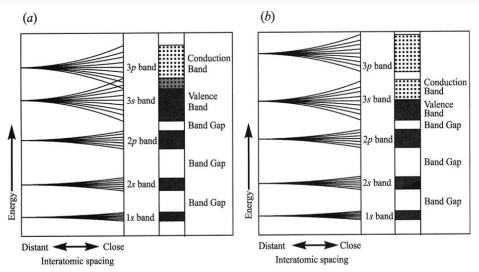
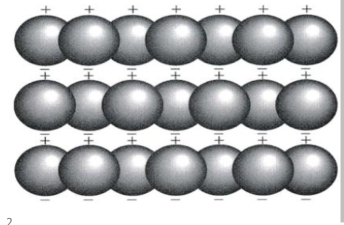
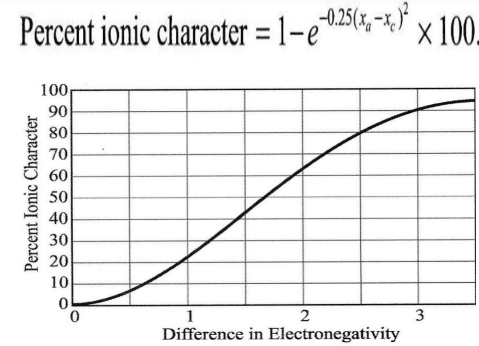
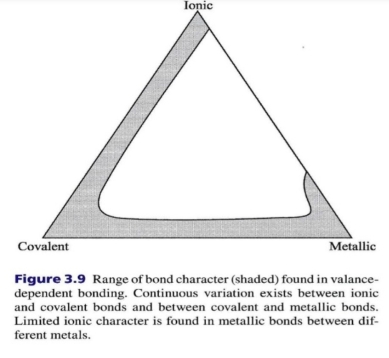
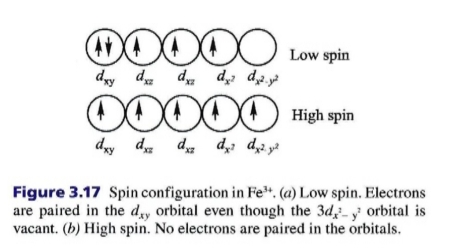
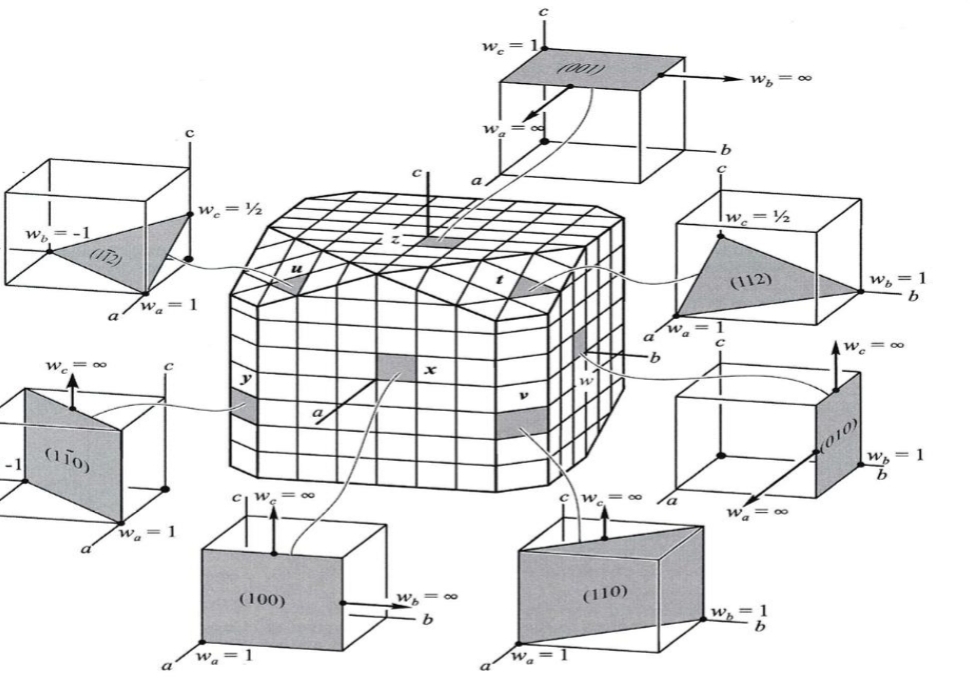
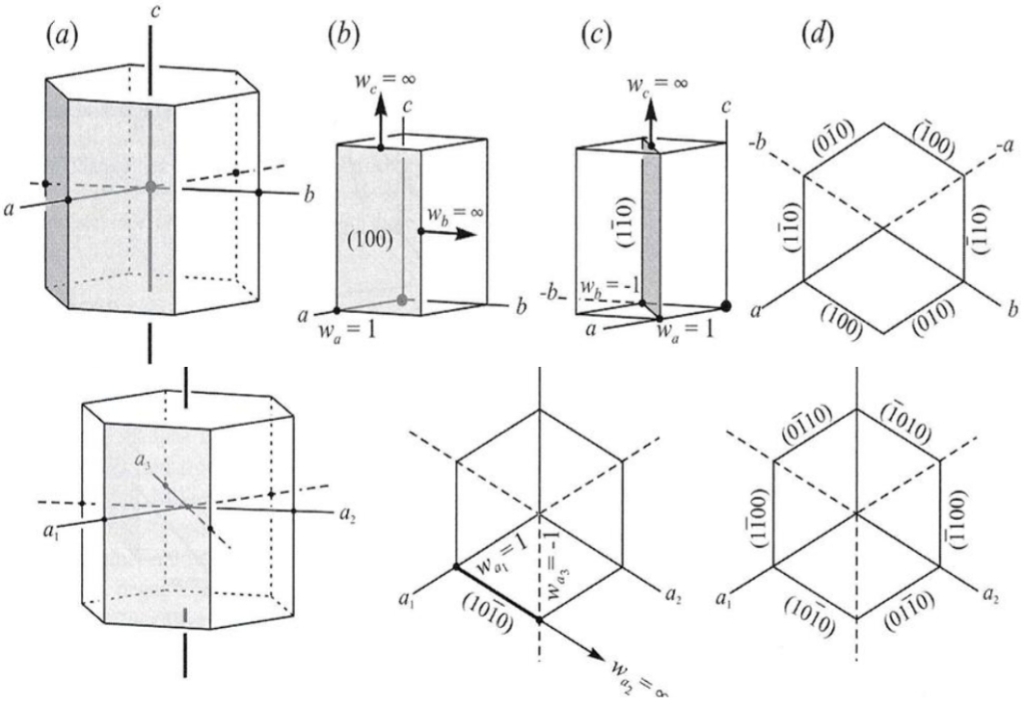
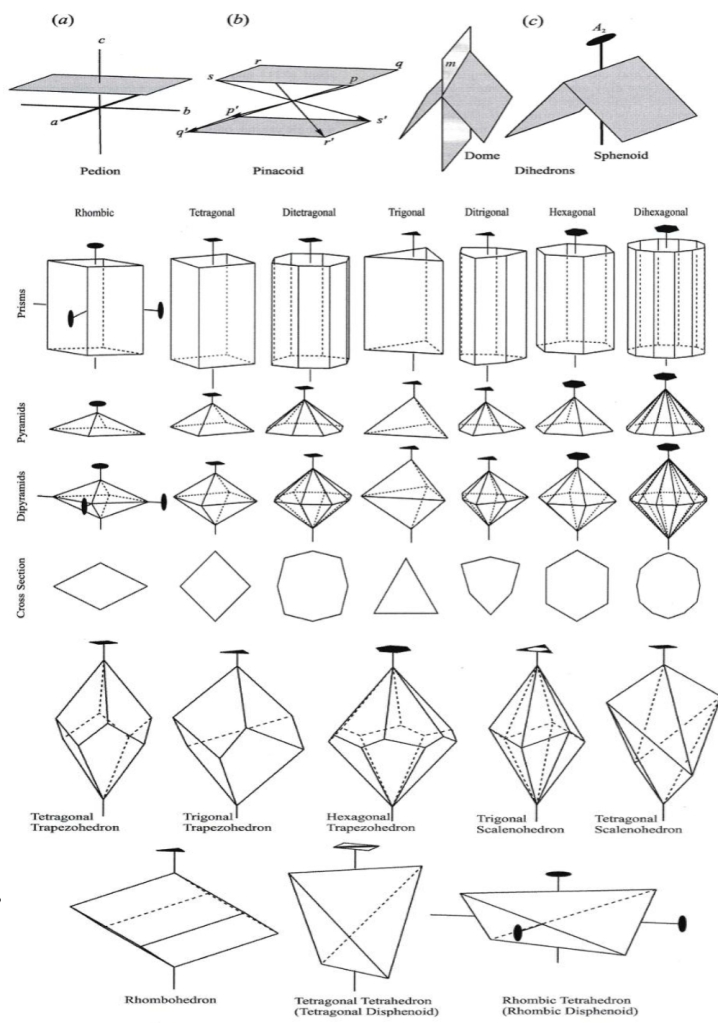
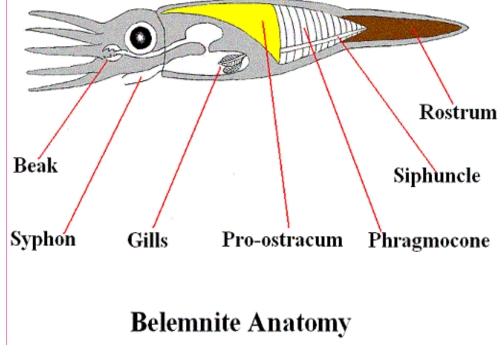
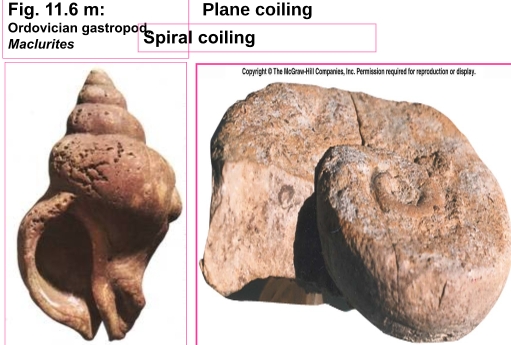
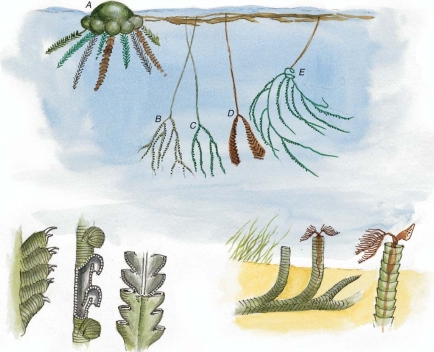
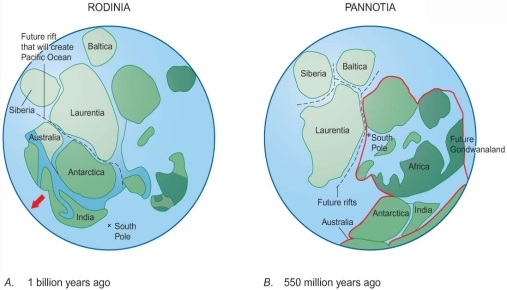
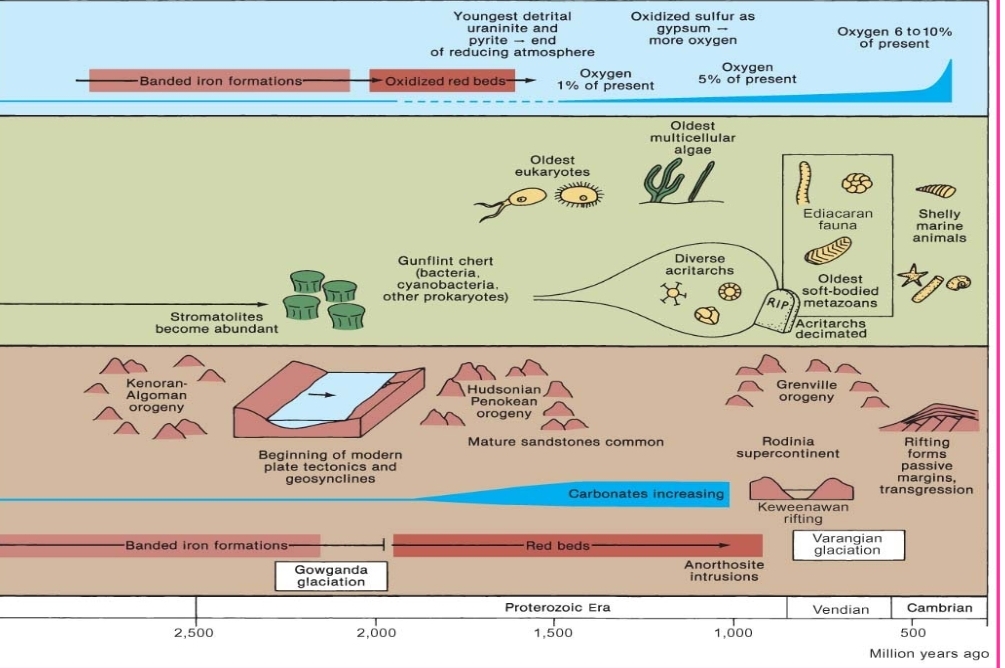
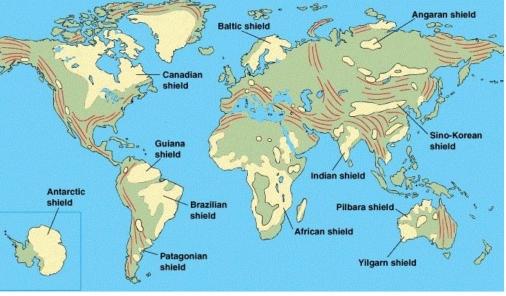
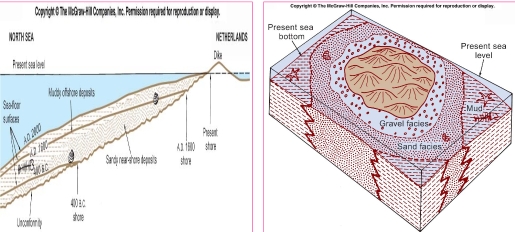
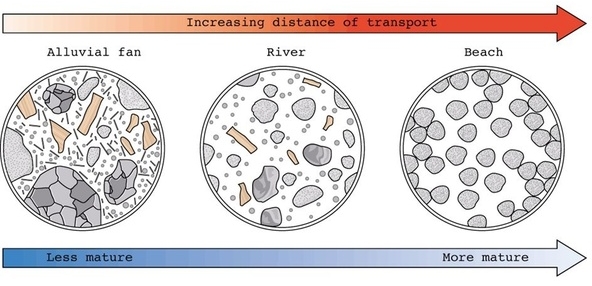
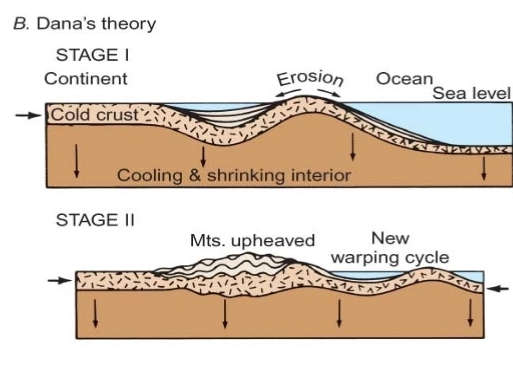
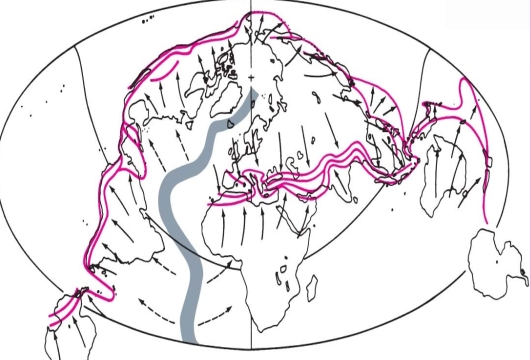
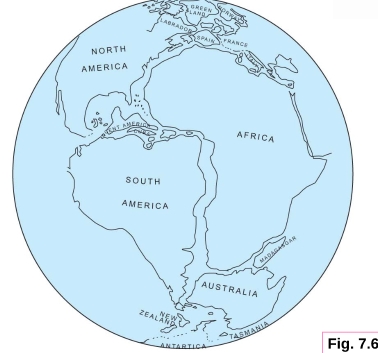
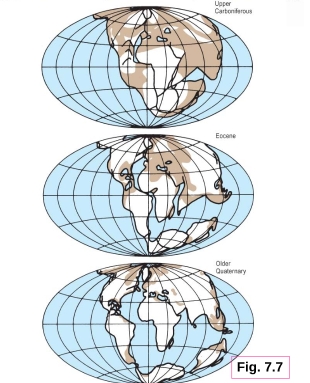
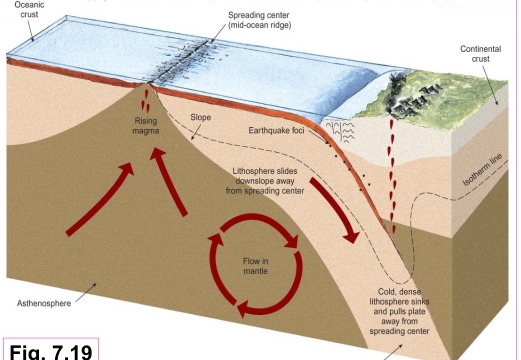
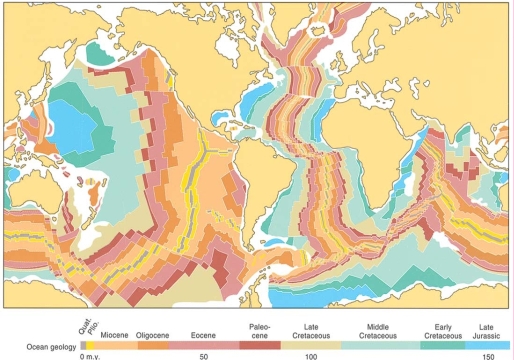
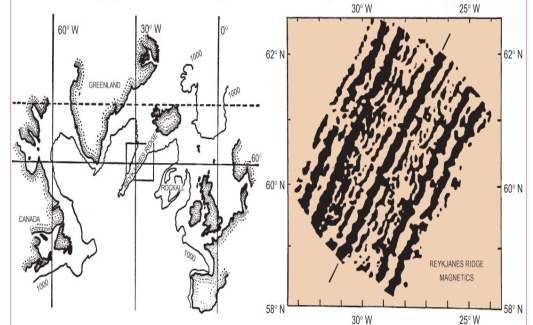
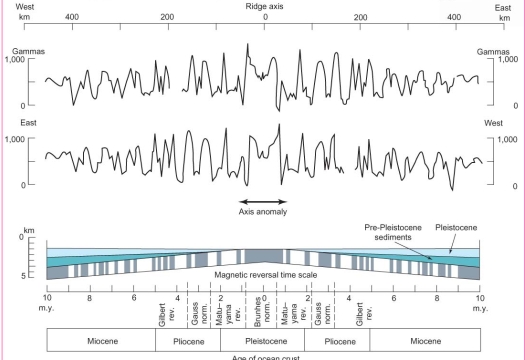
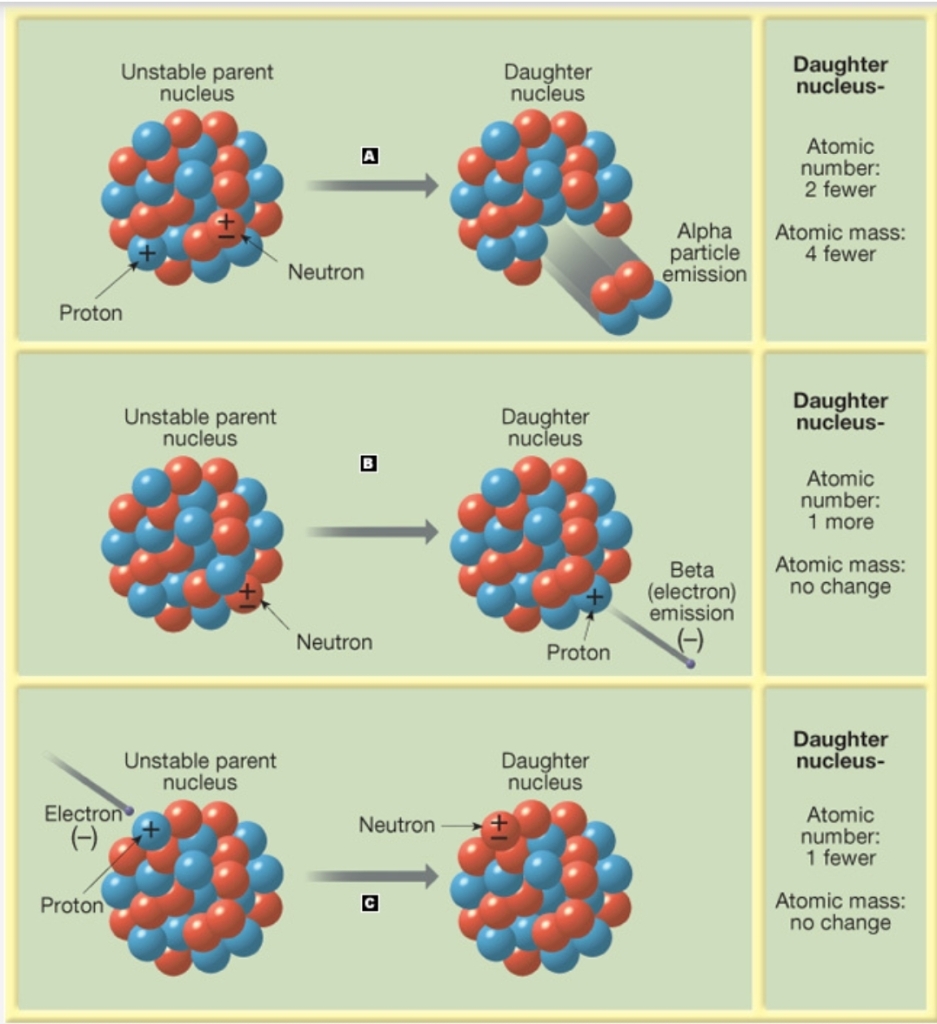
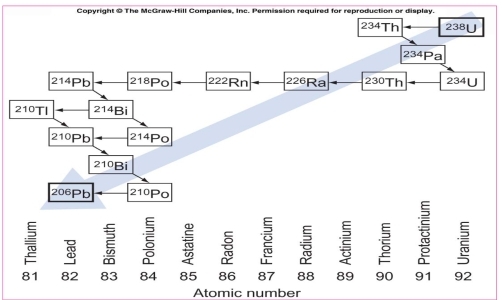
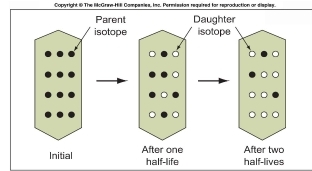
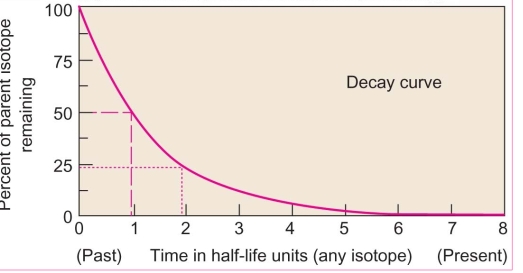
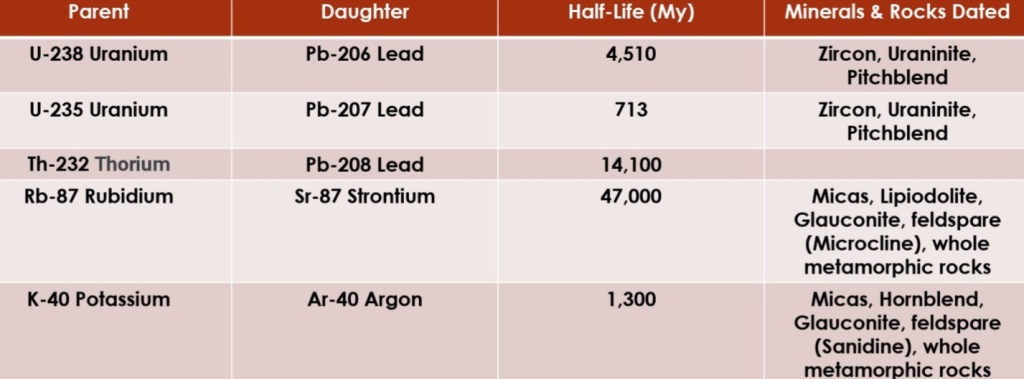
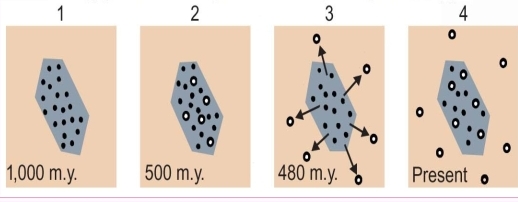
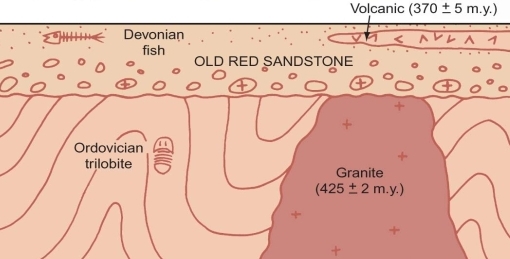
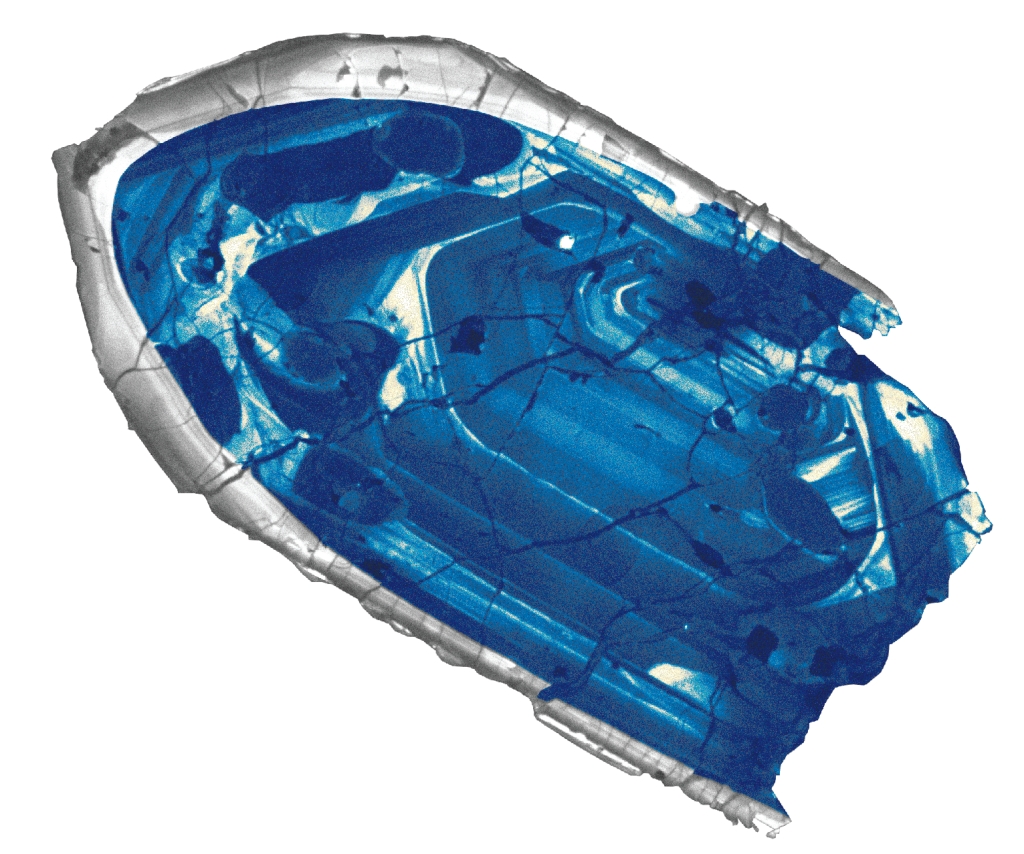
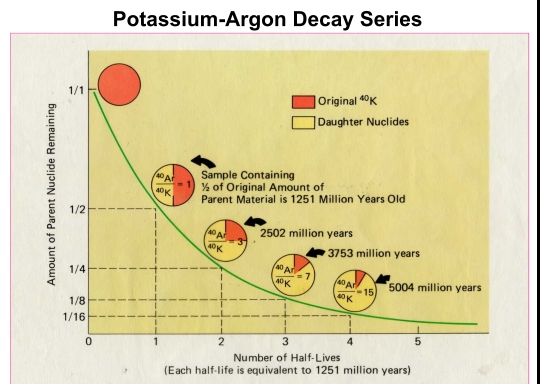
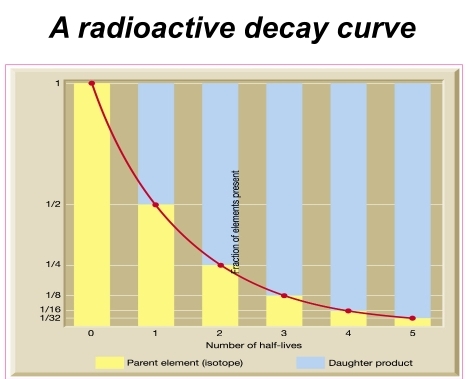
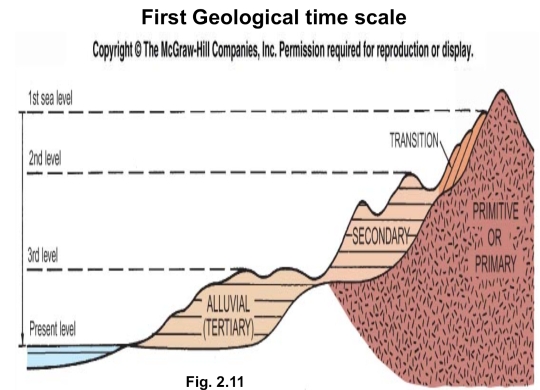
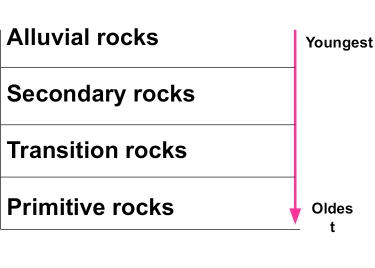
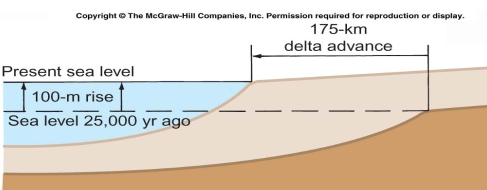
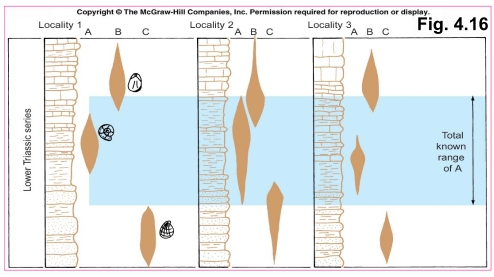
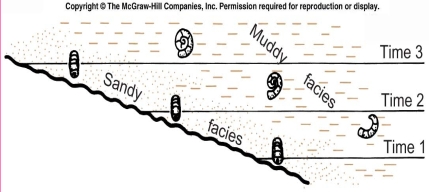
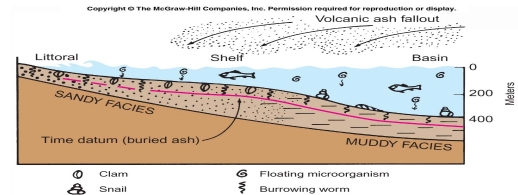
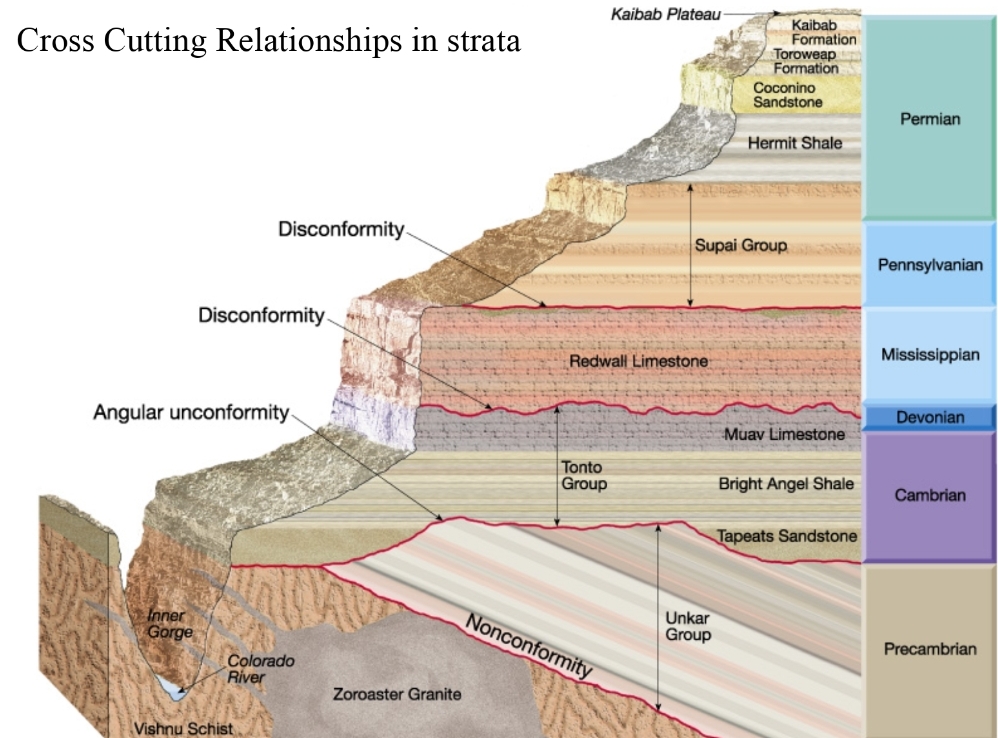
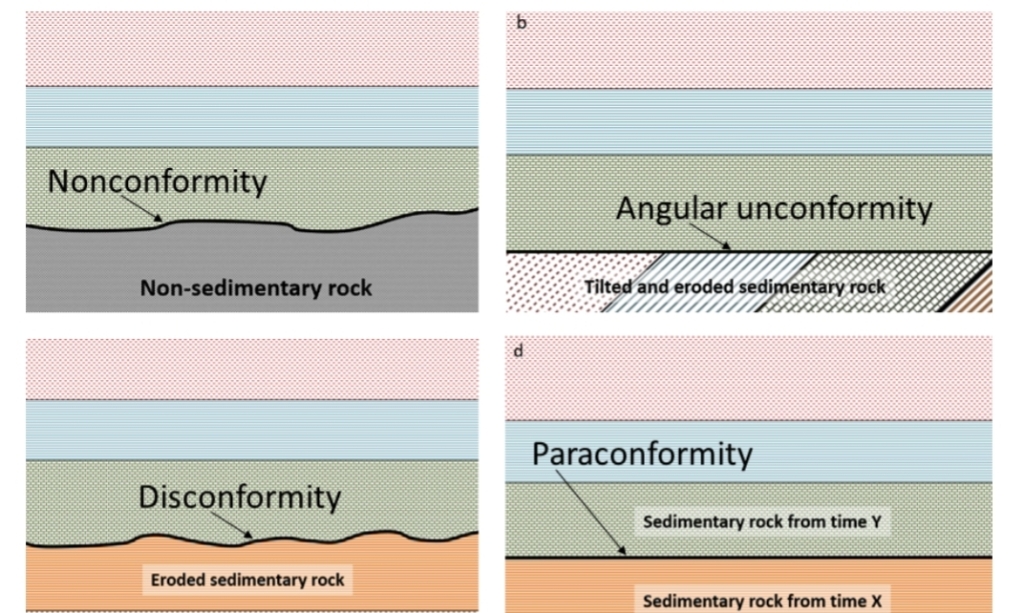
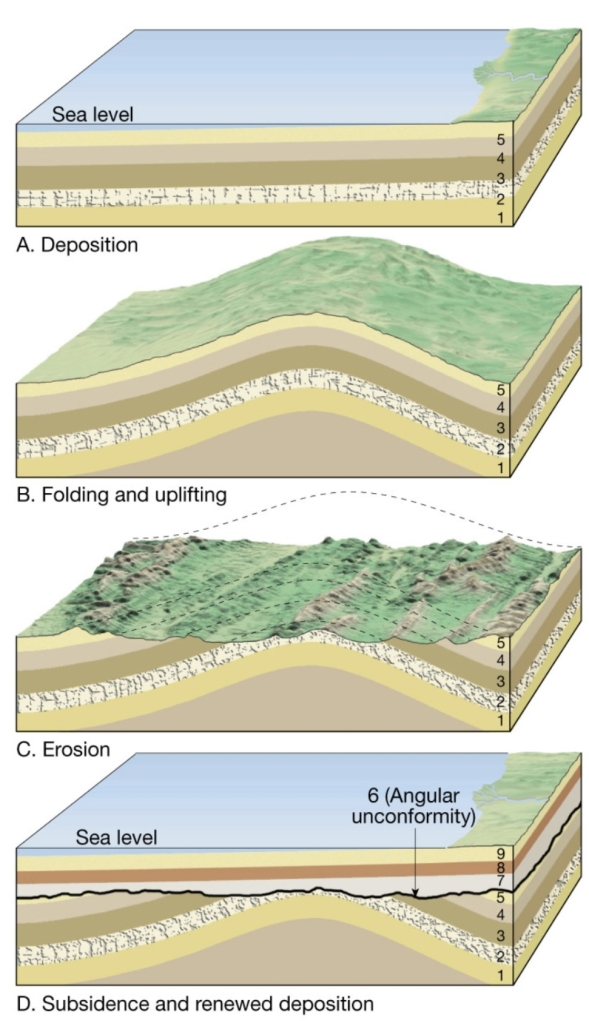
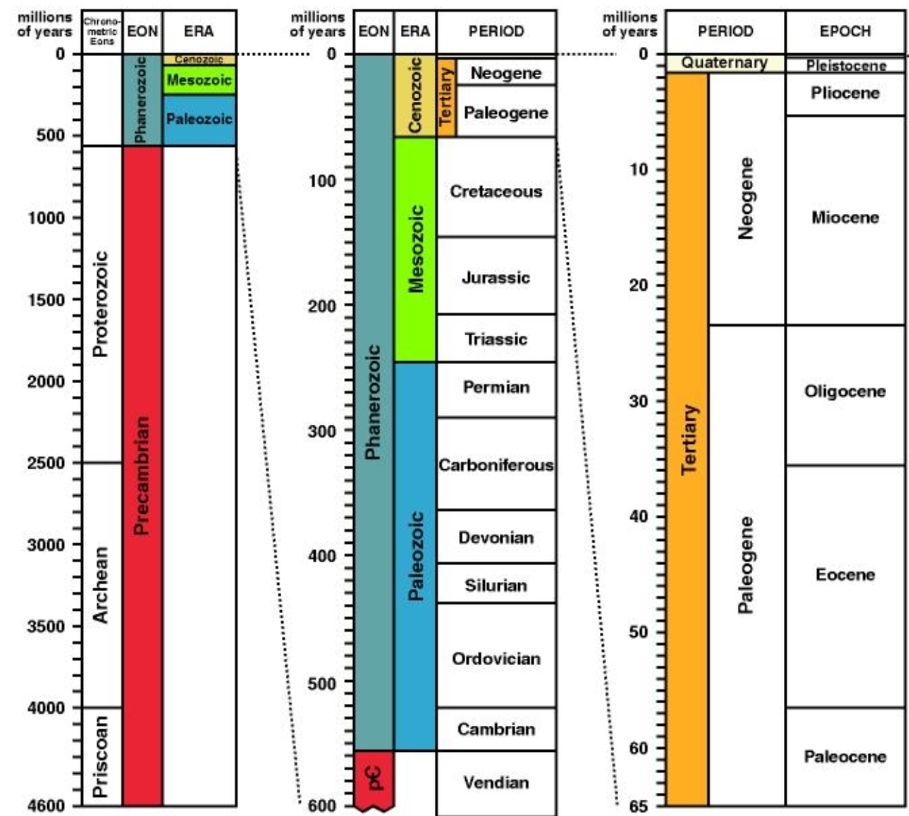
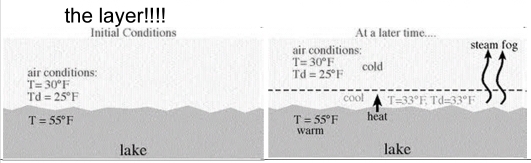
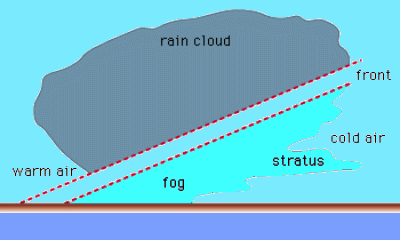
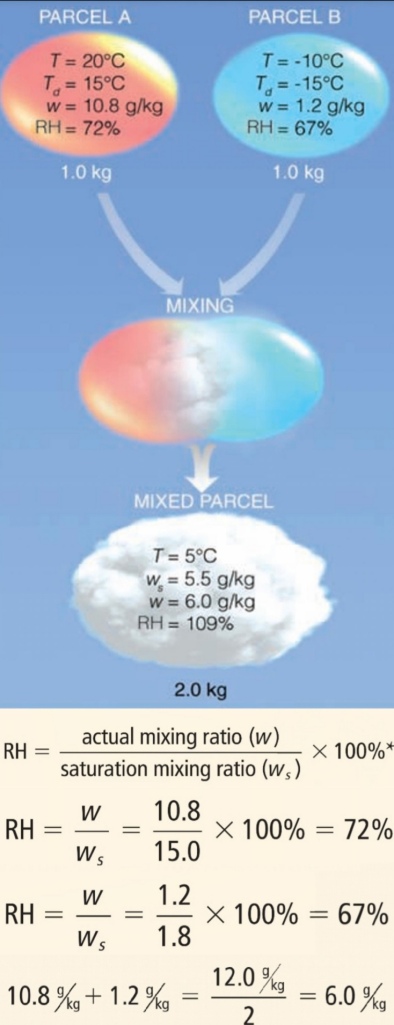
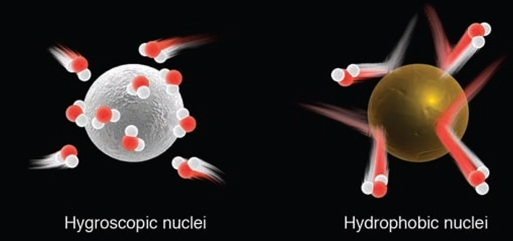
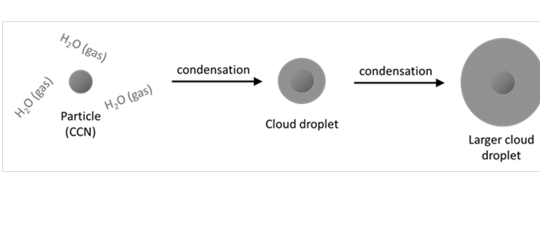
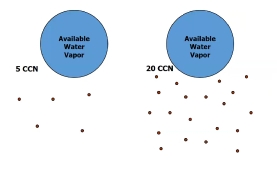
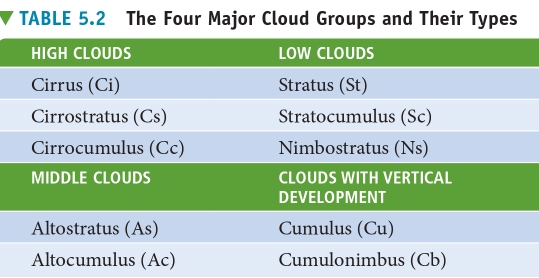
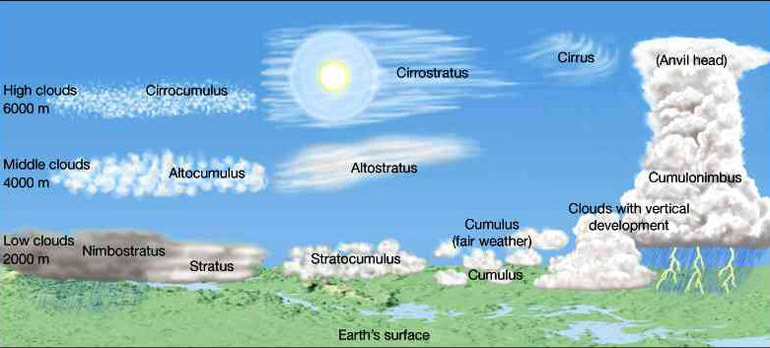
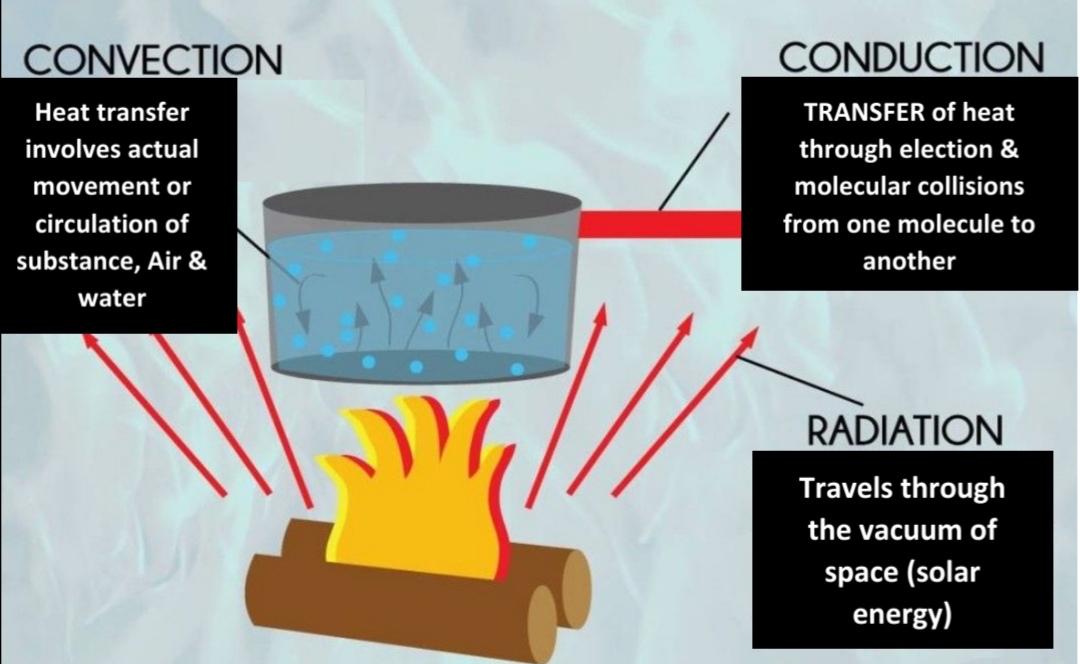
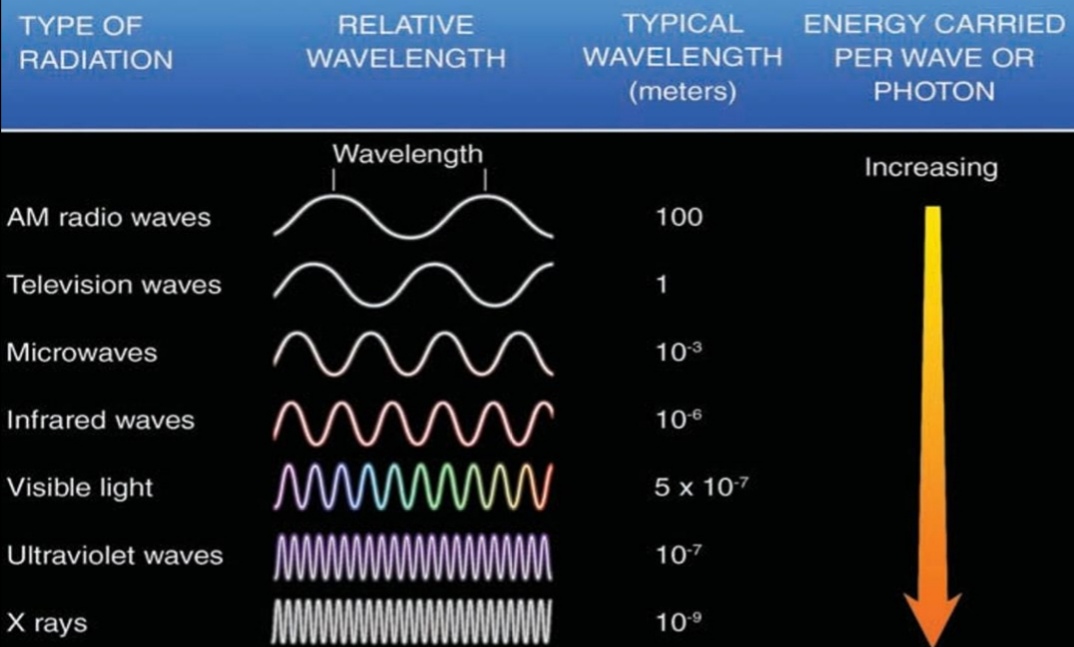
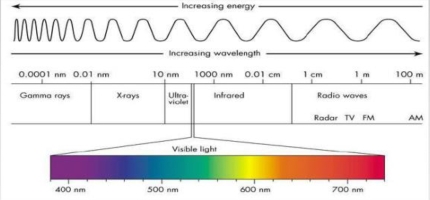
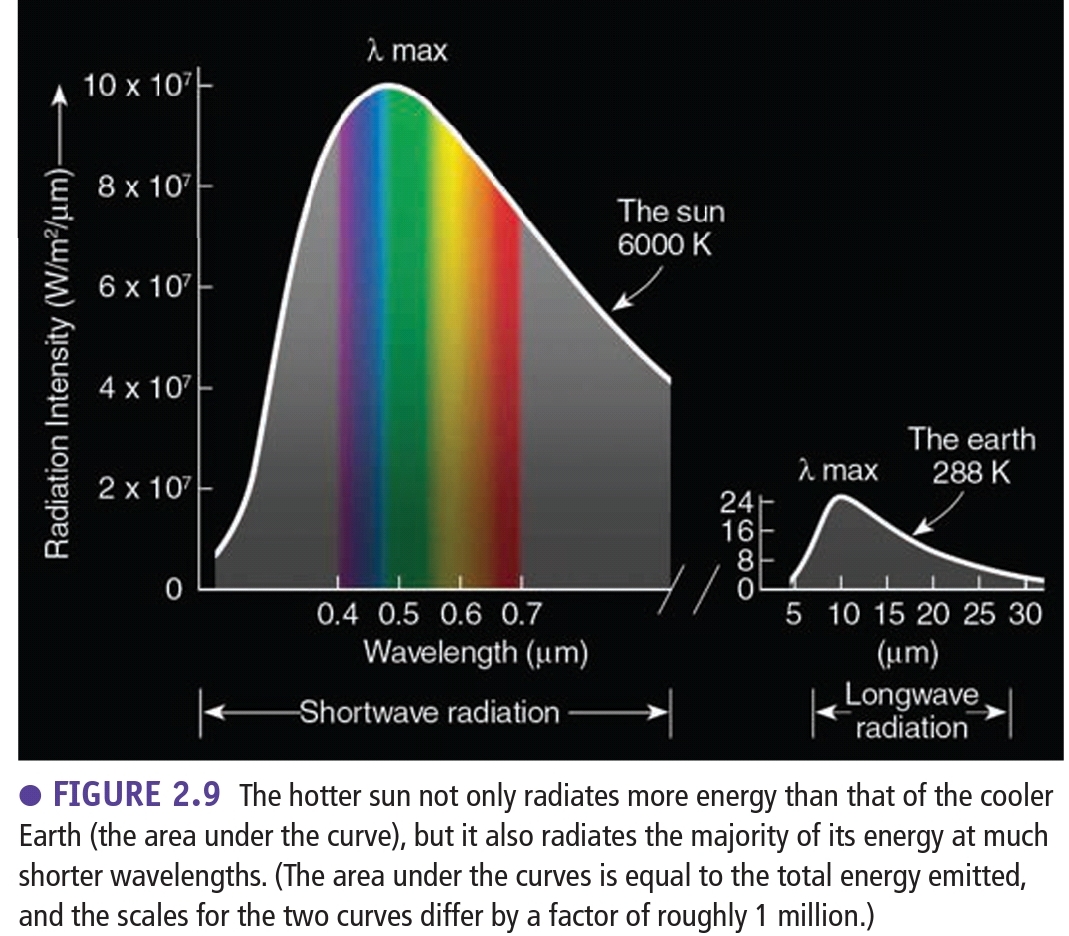
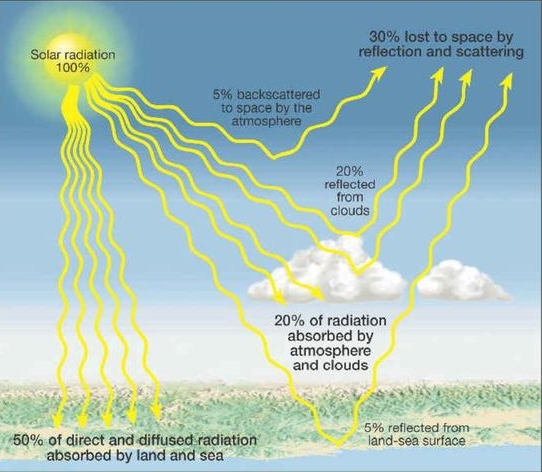
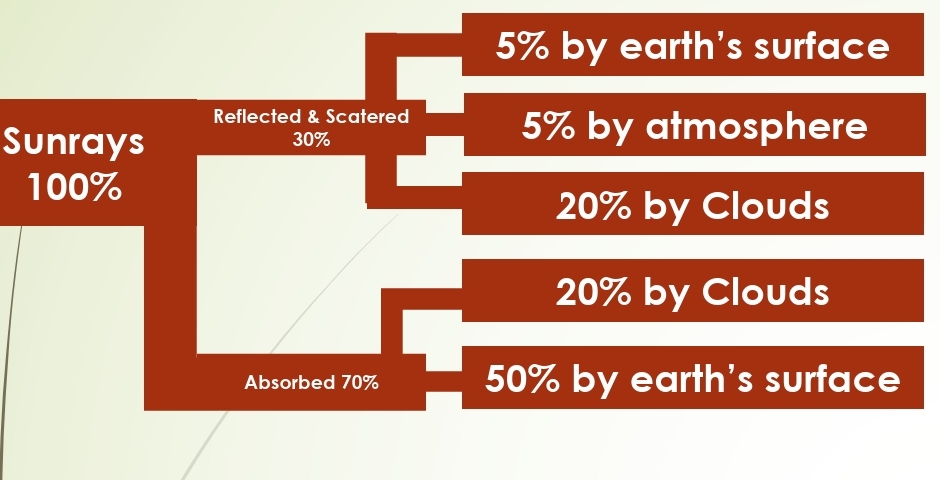
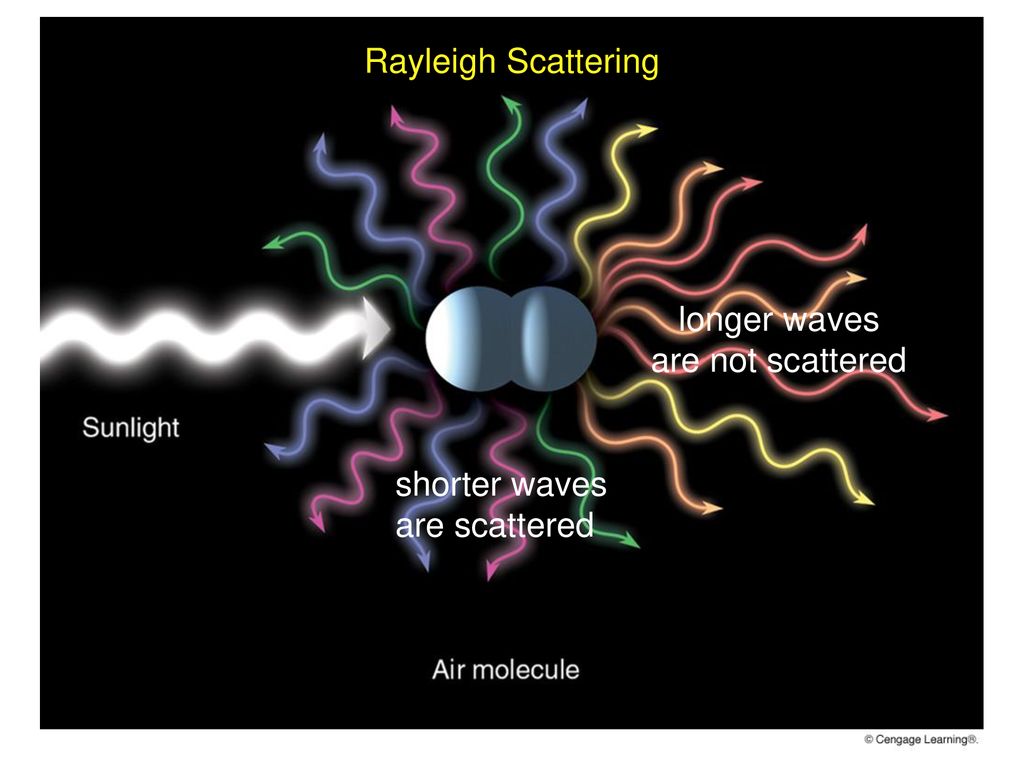
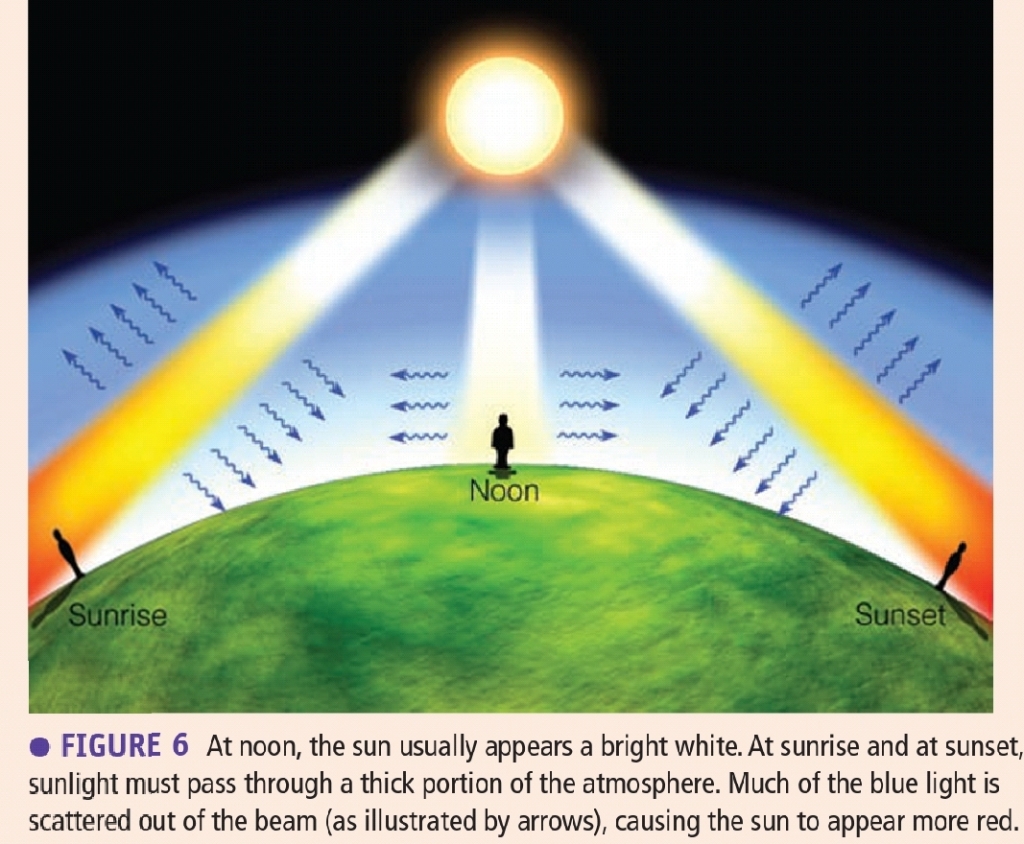
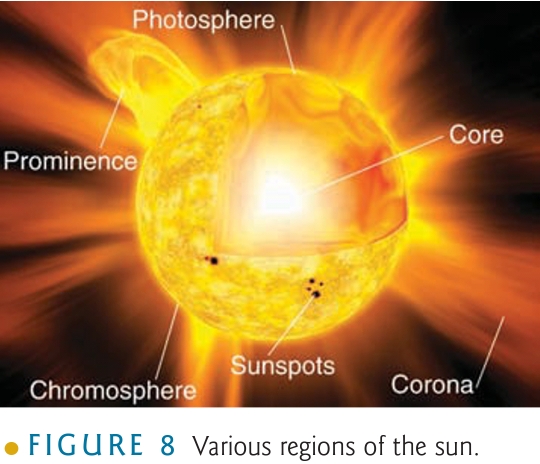
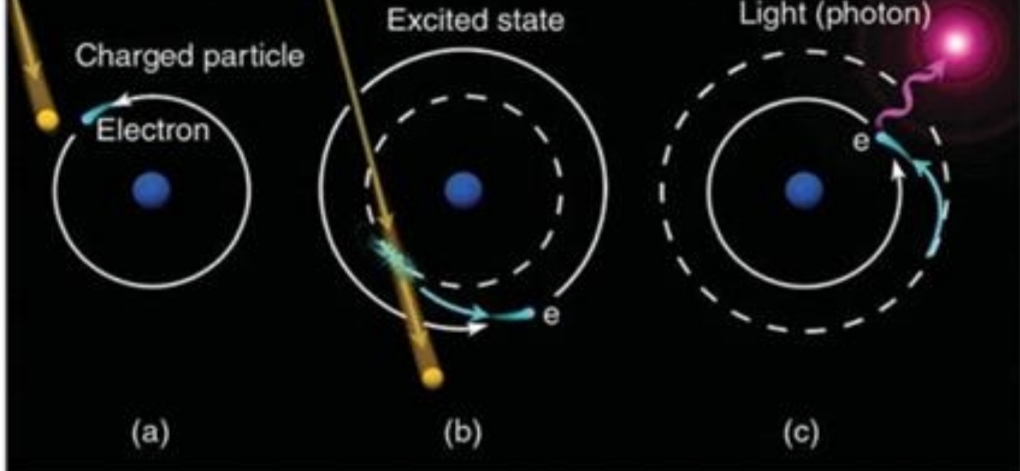
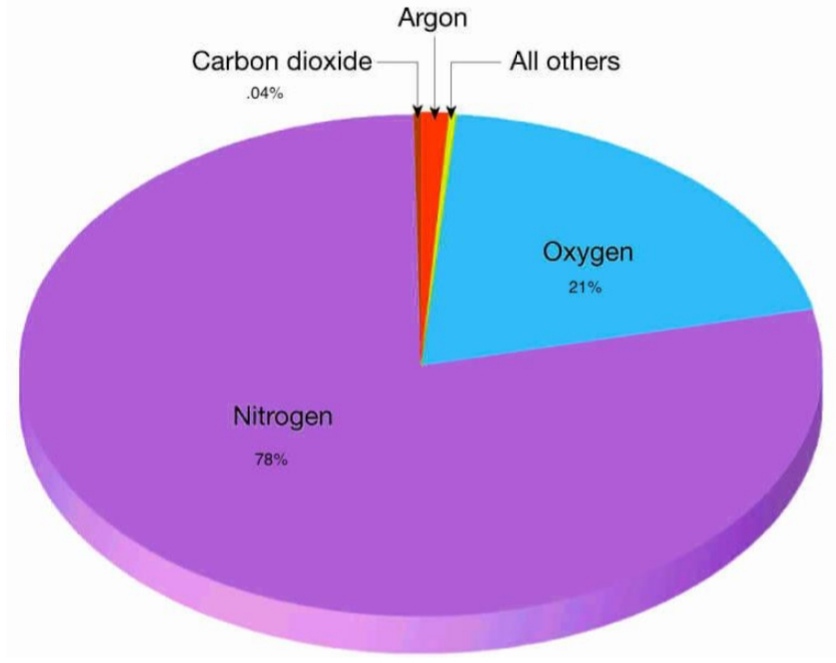
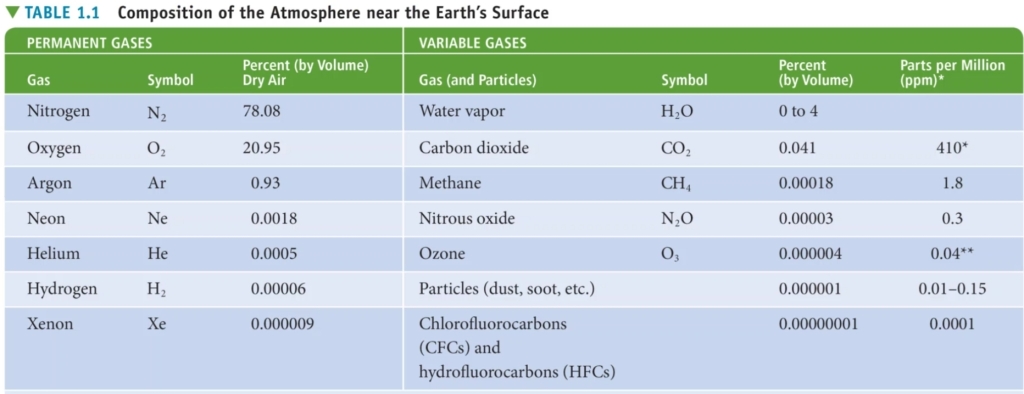
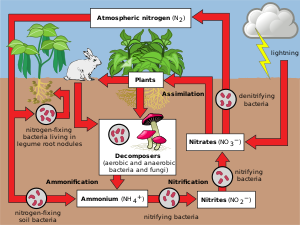
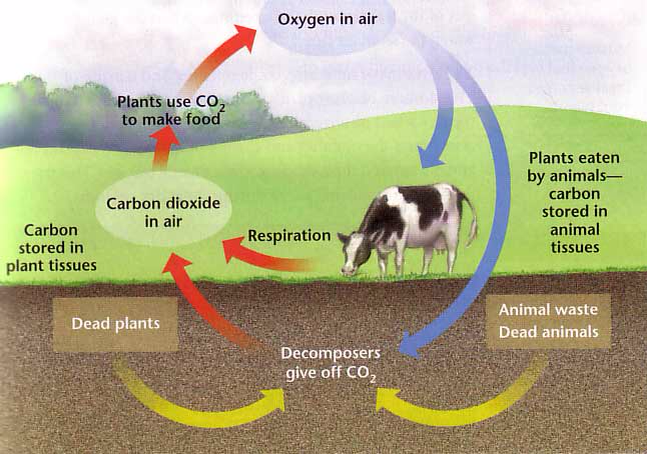
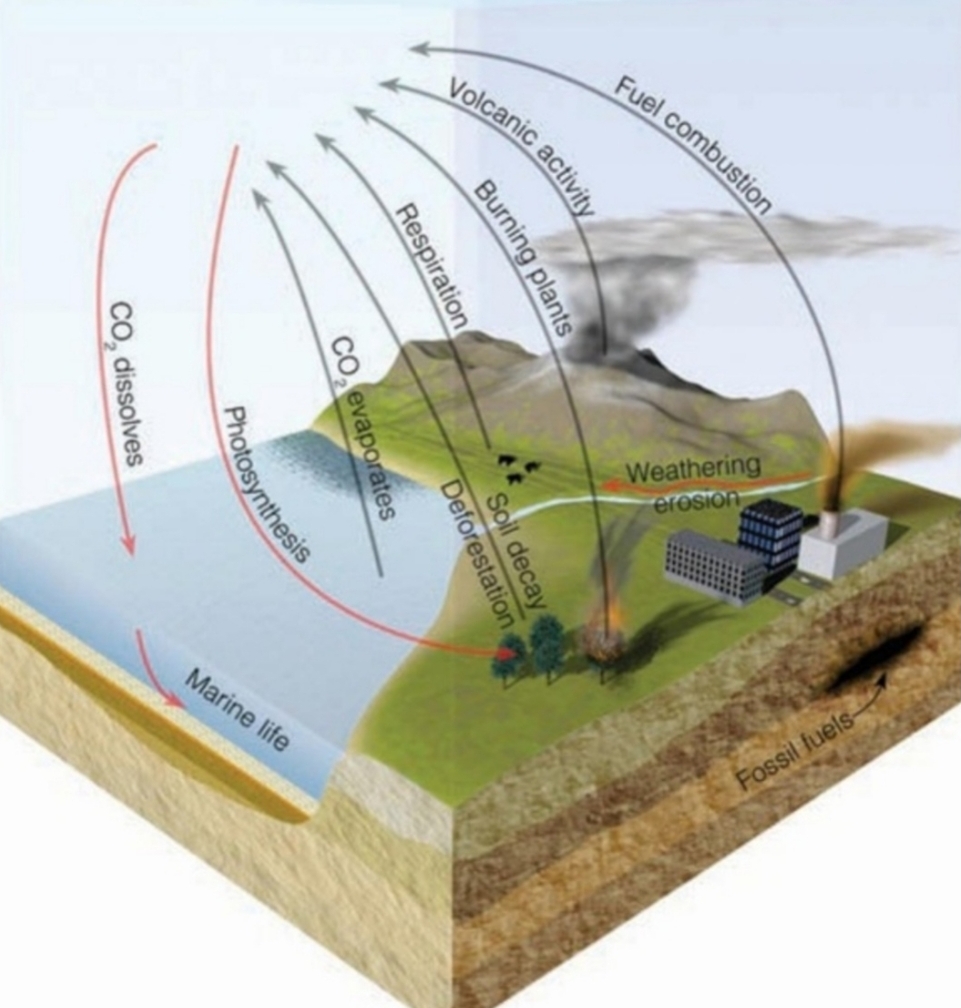
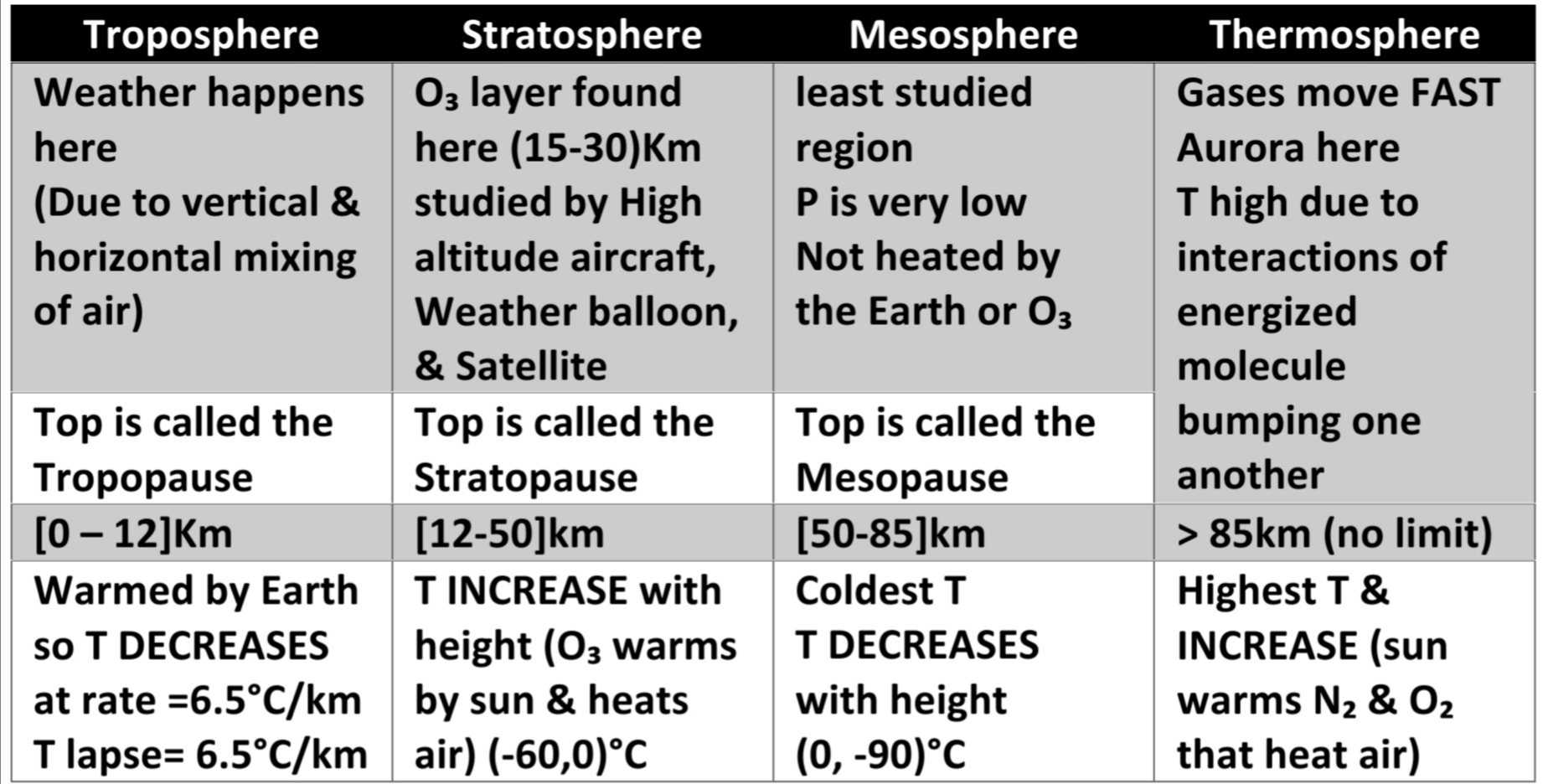
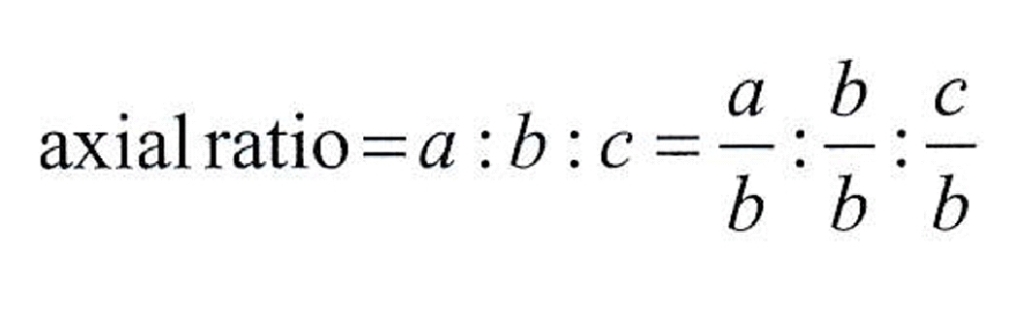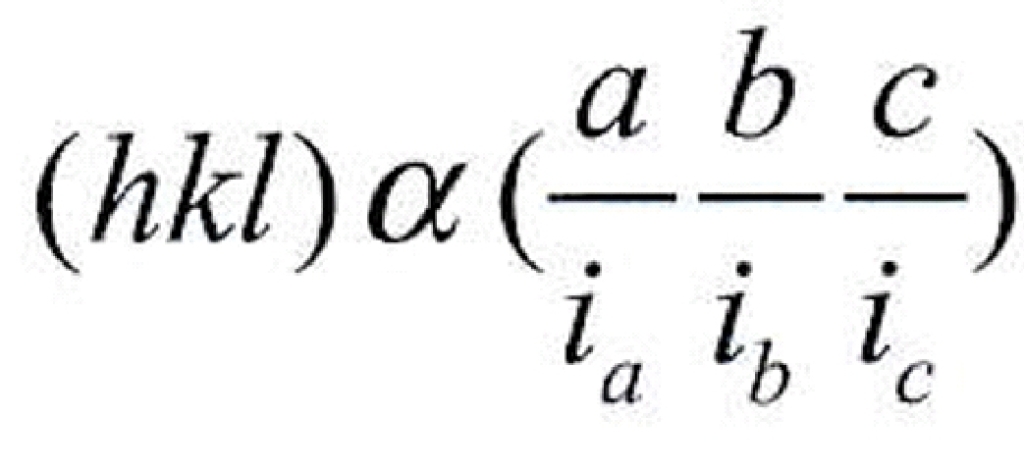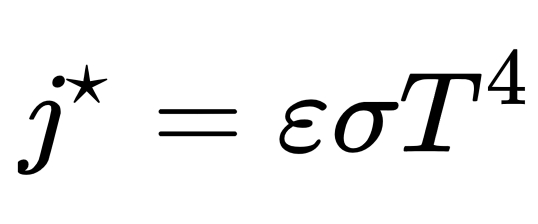Geochemistry & Petrology
Dr. Najel Yaseen
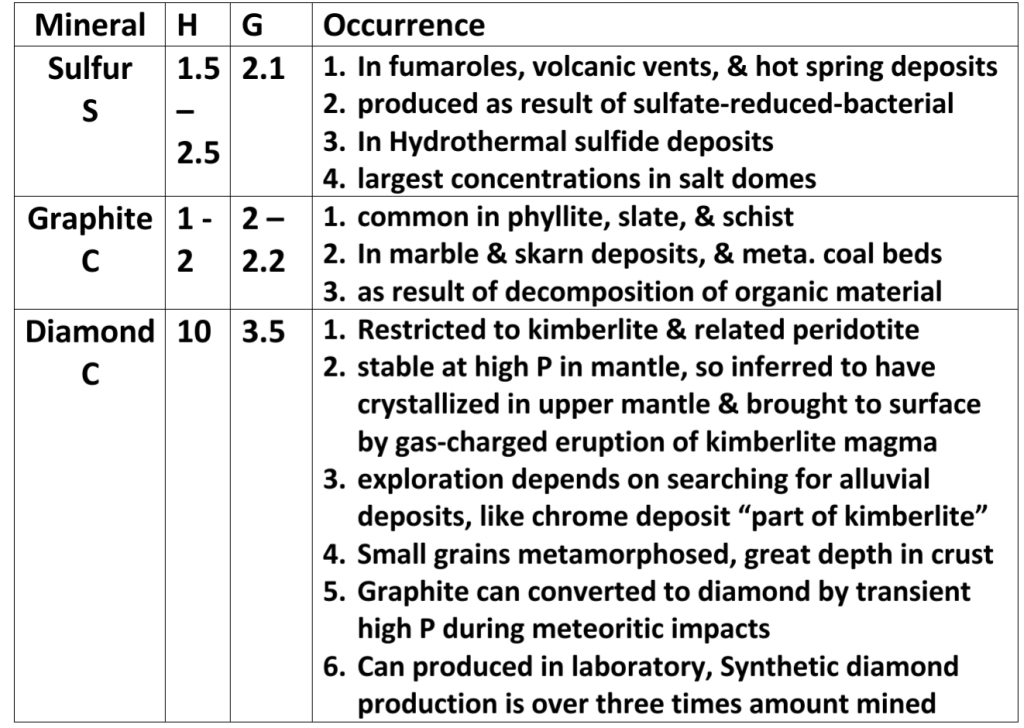
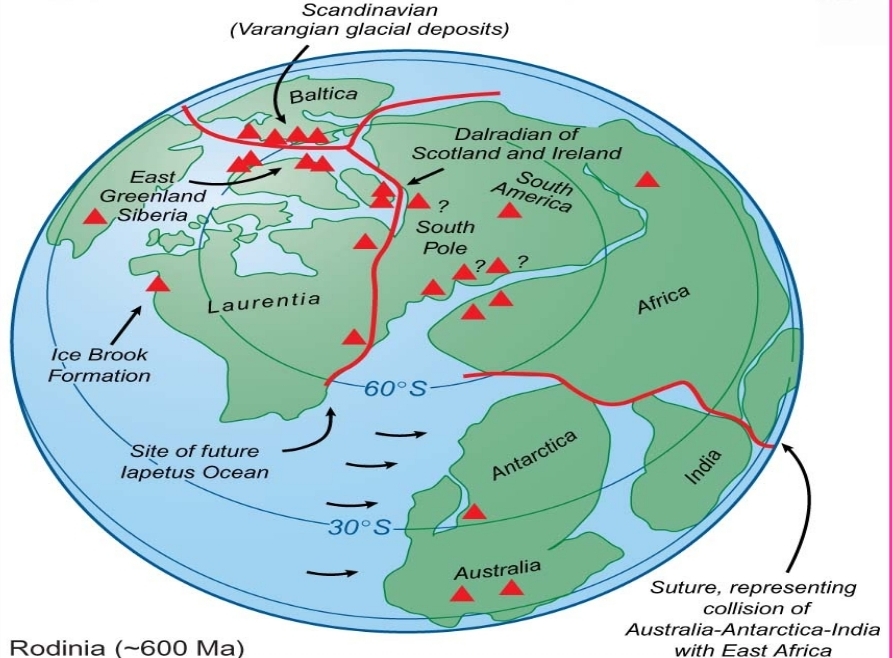
Bayan Alorani, Besan Alshareef and Ola Almasri (2022): Petrography and Geochemistry of Turban Mafic rock, SW Jordan
Istiqlal Odeh (2022): Upgrading of Alkali Feldspar of Humrat and Wadi-Feinan using Magnetic Separation Method
Juman Abu-Touq & Sajeda Al-Rabaya (2023): Petrography and Geochemistry of Thour Mafic Rocks at Al-Quweira Area, SW Jordan
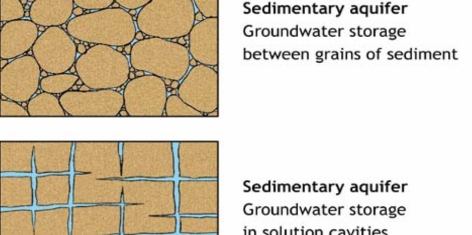
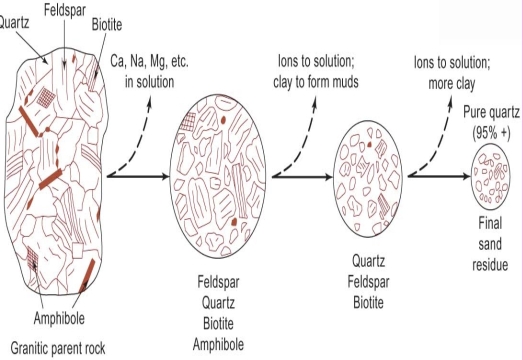
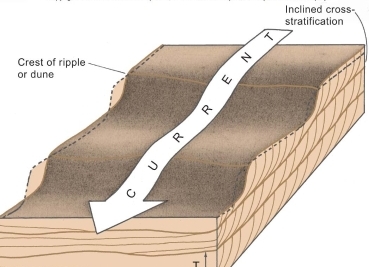
Sara Khalil Al-Haj (2023): Heavy mineral content in Dubaydib sandstone formation
Shaas N Hamdan (2022): Petrology, Petrography, Petrogenesis, Geochemistry and Geothermobarometry of Thour Diorite Unit — Al-Quweira Area / Southern Jordan
Hydrogeology & Climate
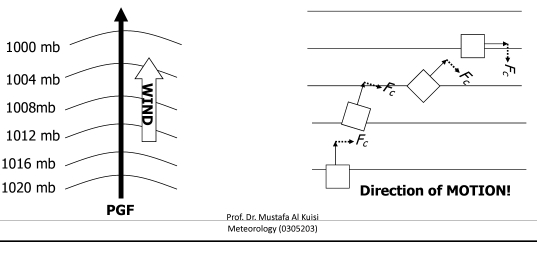
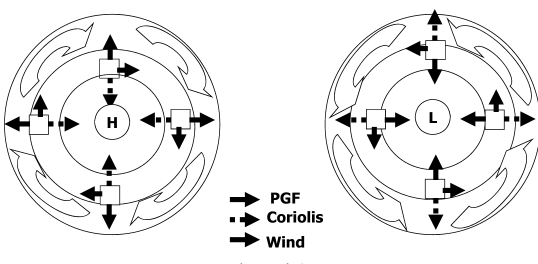
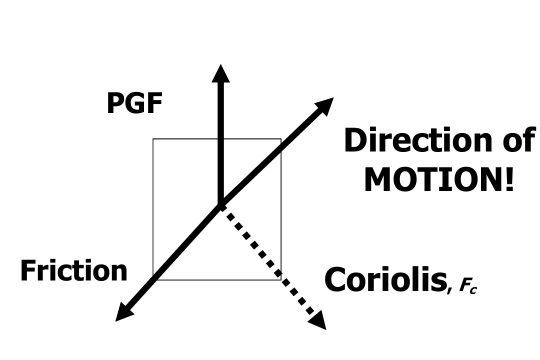
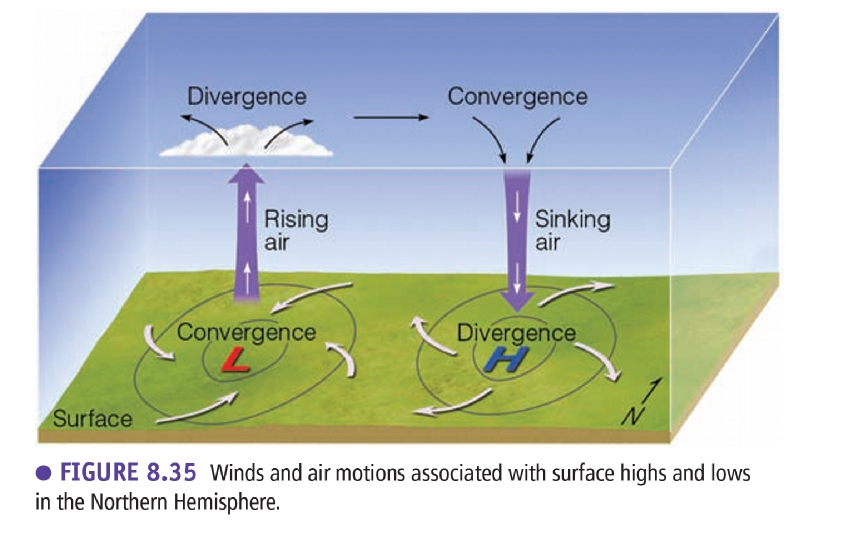
Prof.Dr. Mustafa Al Kuisi
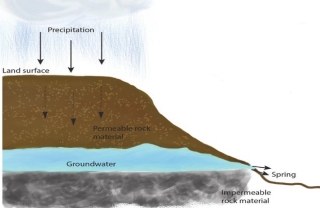
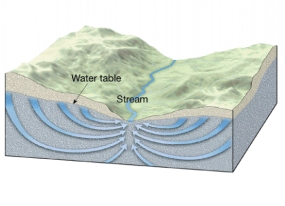
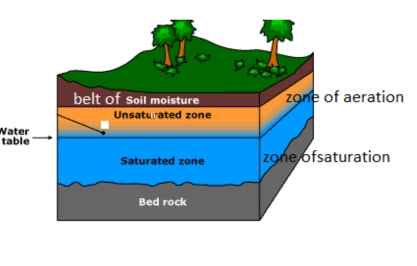
Aisha Al-Shaikh, Sarah Hamasha, Reham Al-Khatib (2023): Water Quality Variations and Hydrochemical Characteristics of Groundwater in Dhuleil Area
Anas Hamdan, Mohammad Mahsere & Mohammad Abulawi (2023): Assessment of Groundwater Vulnerability using COP Model and GIS for Greater Amman area
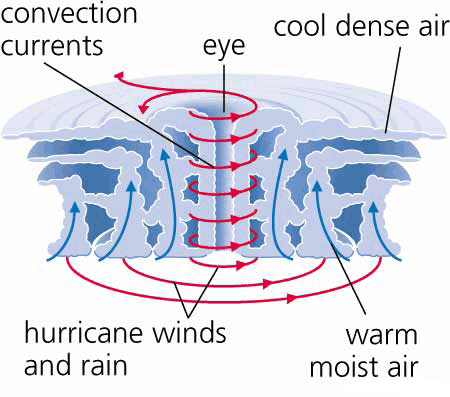
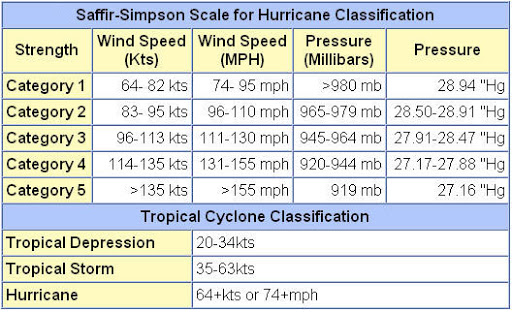
Bayan Halima & Reem Al-Toukhi (2023): Air Quality index for Amman, Irbid, and Zarqa Cities
Saja Alwahsh & Fairouz Al-Salti (2023): Urbanization Impacts on Flood Risks based on Land Cover Devlopments in Amman
Siham Jaber & Abeer Alhalaby (2023): Groundwater Vulnerability Mapping & Risk Assessment for the susceptibility of groundwater resources to contamination for Greater Amman Area by using DRASTIC method
Environmental Resource
Dr. Khitam Al-Zghol
Alaa Afaneh, Hala Sheqwara & Lamees Baker (2023): Geological Characterization of Uranium Minerals Deposits in Central Jordan
Geophysics
Dr. Mu’ayyad Al Hseinat
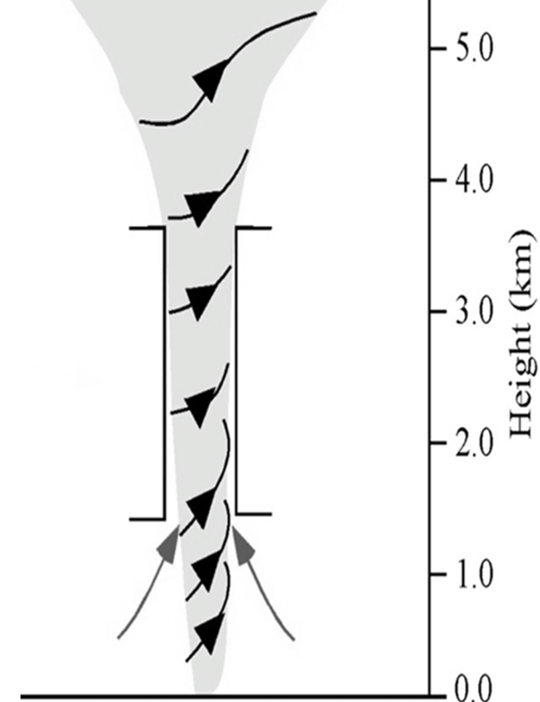
Alaa Al khlaifat & Dania Salameh (2023): Examining the Deformational Style of the Karak Wadi Al-Fayha Fault Zone within the Al-Jafr Region
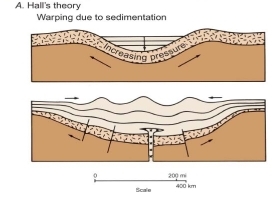
Rand Al-Bdoor & Reema Al-Dhoon (2023): Seismic Reflection Investigation of Al-Sirhan Development Area, southeastern Jordan
Reham Ibrahim & Batool Alazzeh (2023): Examining the thickness of Upper Cretaceous deposits in selective places in Jordan (Jafr, Sirhan, Hamza exploration areas)
Geoarchaeology & Geotourism
Dr. Ahmad Smadi
Iman Mohammad, Ayat Wael & Mais Al-sheikh (2022): Geoarchaeology – Geotourism (Umm Qais Site)“Columns & El-Cardo” (Main Street)
مشروع التخرج هو تدريب على البحث العلمي او بحث علمي يقوم به الطالب في احد فصول التخرج حيث يقوم بتحديد فكرة بحث او مشروع وبكثير من الاحيان اقتباس الفكرة من احد دكاترة واساتذة القسم ثم يقوم بتطبيق معرفته الجيولوجية وربطها في معارف اخرى اذا لزم الامر بوسطة العمل الميداني والمخبري للوصول الى نتائج تتفق مع اهداف فكرته البحثية ثم تفسيرها ثم كتابة تقرير في الدراسة التي قام بها (وهذا الجزء هو المنشور في هذه الصفحة بهدف الاطلاع على تجارب الطلبة السابقين للتعلم منها بكيفية الكتابة العلمية بعد تعديل الدكاترة الاخير)
ملاحظات ونصائح من اخطاء متكررة
* معظم التقارير في هذه الصفحة تنسيقها تالف لكن تسلسلها جيد (حتى الان).
* اذا كان لديك فكرة لبحث ما يفضل ان تبادر بها وان تعمل على تطبيقها عوضاً عن الاعتماد على الدكاترة بالفكرة.
* يتوجب مراعاة اهتمامك في اختيار اي ميدان تريد العمل به، فمثلا اذا كنت تميل للجيوكيمياء عليك اختيار احد الدكاترة المختصين بالجيوكيمياء وموضوع مختص بالصخور والجيوكيمياء وغير ذلك سيصبح ارهاق شديد عليك وضغط نفسي لا يحتمل.
* يمكن القيام ببحث التخرج بمفردك ويمكن القيام به بمجموعات كحد اقصى 4 طلاب، يجب ان تتفقوا مع بعضكم البعض قبل قبول العمل مع بعضكم البعض.
* عندما تذهب الى دكتور ليعطيك فكرة لتعمل عليها ويشرح لك جزء منها استمع له جيدا، وعند خروجك من مكتبه ابحث عن الشيء الذي قاله لك وتأكد انه من ضمن اهتمامك ولا تقبل الفكرة الا اذا كنت تفهم شرح الدكتور لها وانها تناسب قدراتك وفهمك للتخصص لتجنب الضغط النفسي.
* اثناء اجراء خطوات البحث بالعمل الميداني او المخبري سجل كل شيء مباشرة على هاتفك او على دفتر صغير، ادق الملاحظات ولا تترك كل شيء لوقت كتابة التقرير لانك لن تعلم حينها ما الذي فعلته وكيف ستبدأ بالكتابة.
* اثناء الكتابة، اي معلومة تحصل عليها من اي مصدر اياك ان تنقل المعلومة وتترك مصدرها بل انقل المعلومة وانقل المصدر الذي تحصلت به على المعلومة وغير ذلك سيصبح توثيق ما كتبته عملية معقدة جدا وغالبا لن تستطيع القيام بها بمفردك بل تحتاج مساعدة دكتور او مكتبة او احد الـ “نيردات” الملمين جدا في المجال الذي تبحث به، لذا تجنب هذا الامر.
كتاب الكتابة العلمية لغير الناطقين باللغة الانجليزية:
Science Research Writing for Non-native speakers of English